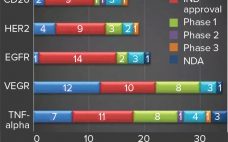
Since the 1986 approval of the first recombinant therapeutic antibody, OKT3, biopharmaceuticals have become a large percentage of overall pharmaceutical company revenue. In 2018, sales of the top five selling recombinant proteins — Humira (adalimumab, AbbVie), Keytruda (pembrolizumab, Merck), Herceptin (trastuzumab, Genentech), Enbrel (etanercept, Amgen), and Avastin (bevacizumab, Genentech), all antibodies — totaled over US$48 billion. The compound annual growth rate (CAGR) for antibodies revenue was about 20% from 2004 to 2014. Those products include naked monoclonal antibodies (MAbs), Fc-fusion…
Business
Cell Banking for Cell and Gene Therapy: Regulatory, Ethical, and Scientific Considerations
Regenerative medicine holds great potential for human disease management, with hundreds of cell and gene therapy (CGT) products for tissue/organ reconstitution or replacement in different stages of development and clinical testing for toxicity, safety, and efficacy. For example, currently more than 60 CGTs have marketing authorization (although many with only conditional approval) from central regulatory agencies worldwide (1). Those products are treating conditions such as hematopoietic malignancies, immunological disorders, and cartilage disorders. Most of those treatments use culture-expanded autologous or…
eBook: Joining Forces — Industry Collaborations Toward BioProcess Success
Companies in the biopharmaceutical industry increasingly are working together to solve the many challenges of product/process development and biomanufacturing. Suppliers seek end-user help in refining technologies; academics and small innovators attract the financing and business acumen of large companies; equal partners share in technological problem-solving; and sponsors engage the development expertise of contract research and manufacturing organizations. Other examples of biopharma industry collaborations abound, too. Citing critical examples from the September 2019 BioProcess International East Conference in Boston, MA, this…
eBook: Speed to IND — Balancing Risk and Reward
With so many biopharmaceuticals obtaining breakthrough or fast-track designations, companies that use accelerated strategies to be first in human studies can be left with significant quality and manufacturing challenges that must be solved later on. Despite regulatory encouragement to create solid design spaces and define parameters according to quality by design (QbD), those may go by the wayside given the pressures of speed. The reward is the investigational new drug (IND) application itself — but if companies lock in subpar…
Making Media a Priority: An Interview with Susan Riley of Advanced Bioprocessing
Susan Riley is vice president and general manager of Advanced Bioprocessing. It’s been a year since Thermo Fisher Scientific’s acquisition of the Advanced Bioprocessing business from Becton Dickinson (BD). Why did Thermo Fisher see the Advanced Bioprocessing (AB) business as a good fit with its life-science offerings? AB has a significant portfolio in premium supplements for cell culture and microbial fermentation. The AB business was seen as a good fit for several reasons: It goes hand-in-glove with Gibco media, for…
From Supplying Components to Providing Total Solutions: Overviewing Supplier Side Capabilities
Only a thin line now separates biopharmaceutical manufacturers and suppliers because the latter are increasingly becoming the process knowledge owners in the biopharmaceutical industry. As a result, suppliers are racing to become the most efficient “total solutions” provider. In the 1990s, leading players in the industry such as Pall, Millipore, and Sartorius all supplied membrane filters for upstream and, to some extent, downstream processes with their crossflow and final filtration offerings. Pharmacia (which became GE Healthcare) was the major force…
The First Quantitative Industry Assessment of Single-Use System (SUS) Reliability: Raising the Bar for BPSA’s Value to Industry
Over the past 24 months, the leadership of the Bio-Process Systems Alliance (BPSA) has initiated a pilot program to demonstrate the joint feasibility of collecting and sharing pertinent industry business data. We began with “quality-complaint data” centered on the premise that product complaints are tightly associated with product-quality defects in single-use systems (SUSs). This approach can be viewed as “risk assessment 101.” BPSA’s Representation of the Single-Use Industry BPSA is an industry organization primarily comprising drug manufacturers, single-use system suppliers,…
Streamlining the Path to Clinical Manufacturing
Careful consideration of lot size is crucial for multiyear success of a cell therapy business. RoosterBio’s product design and acceleration business works with the company’s customers to help make plans that are appropriate for their stages of product and clinical development. We use the following major considerations in creating a sound multiyear strategy with intermediate milestones: Understand what the future looks like and work backward Use reasonable to conservative assumptions to estimate a range of needs Invest in the right…
Demystifying the Patent Search Process — Revisited
One of the first steps toward understanding how to patent an invention, invalidate someone else’s, or enter a new area of research is conducting a patent search. Knowing whether your invention is similar to those that already have been reported is critical before applying for a patent or beginning research. If your invention has been patented already or has been described or published anywhere in the world, then it is considered “prior art,” and your patent will be denied. Numerous…
Biopharmaceutical Growth in China: Bioprocessing Challenges Are Creating CMO Opportunities
In the past decade, the world has seen rapid growth of more than 15% in China’s biopharmaceutical market (1). According to the second edition of BioPlan’s Advances in Biopharmaceutical Technology in China, the most populous country in the world — with the largest patient groups — has a growing economy with its GDP second only to that of the United States. Rapid urbanization and greater access to national healthcare insurance puts China in second place after the United States for…