Since 2003, the mission of BioProcess International has been to connect biopharmaceutical scientists and decision makers to the science, technology, and expertise that can positively influence and improve existing bioprocesses. Our BioProcess International Awards were created in 2012 to mark the magazine’s 10-year anniversary. They allow us to reflect on and help honor the time and investment companies put into researching, developing, and launching biopharmaceutical products, technologies, and services to deliver better, more efficient treatments and increased hope to a…
Search Results for: antibody characterization
Design and Performance of Single-Use, Stirred-Tank Bioreactors
Single-use components and systems have been incorporated into many bioprocesses as an alternative to cleanable, reusable systems. A wide range of publications have detailed the reasons for this trend toward a single-use approach. Justification in many cases comes from process-specific benefits such as increased manufacturing flexibility — especially for contract manufacturing organizations (CMOs) — enhanced sterility assurance, elimination of cleaning, reduced capital investment, faster processing times with increased productivity, faster start-up, and other benefits (1). One critical factor in the…
Biosimilar Therapeutic Monoclonal Antibodies: Gaps in Science Limit Development of an Industry Standard for Their Regulatory Approval, Part 1
Biosimilars are biologically derived pharmaceuticals intended to have clinical similarity to a legally marketed innovator product when that product’s patent or market exclusivity has expired. By contrast with generic small-molecule drugs, clinical performance of a biologic pharmaceutical is a function of its structural complexity and higher-order structure (HOS). Biomanufacturing controls of such complex products cannot fully ensure chemical similarity between an innovator product and putative biosimilar because minor differences in chemical modifications and HOS can significantly alter a product’s safety…
Emerging Technology Trends in Biologics Development: A Contract Development and Manufacturing Perspective
For a contract development and manufacturing organization (CDMO), process development and manufacturing of recombinant proteins must be linked because of tight timelines driven by client expectations. Those are in turn driven by a need for rapid progression to clinical testing. Early in process development, the choice of raw materials needs to reflect existing supply chain and manufacturing infrastructure, but remain suitable for scaling up to meet future needs. One approach is to establish platform processes for a class of molecules…
Outsourcing Trends in Biosimilars Development: A Discussion with Niall Dinwoodie (Charles River Laboratories)
No discussion about the future of the biopharmaceutical industry would be complete without assessing the impact of biosimilars. But such discussions no longer focus on whether biosimilars will enter the market, but rather when and how much market share will they take. The rapid progression of biosimilar candidates in company pipelines and the strong biosimilars research conducted by international organizations are strong indications that if your company is not already working within the biosimilars market, it may already be too…
Quality By Design for Monoclonal Antibodies, Part 2: Process Design Space and Control Strategies
Process design space and control strategy are two fundamental elements of quality by design (QbD) that must be established as part of biopharmaceutical development and regulatory filings. Like all of QbD, they are interconnected and iterative. Both are based on knowledge gained during product and process development — but both need to be in place (in a potentially very limited form) when a company begins to manufacture drug substance for clinical trials. Part 1 of this discussion appears on pages…
Science, Risks, and Regulations: Current Perspectives on Host Cell Protein Analysis and Control
State-of-the-art analytics guide process development by providing companies with thorough understanding, effective removal, suitable control, and comparability assessment after process changes of host cell proteins (HCPs) in recombinant biotechnology products. An array of analytical techniques and approaches can be used to establish control strategies for host cell proteins. Techniques used for HCP characterization and comparability include two-dimensional (2D) gel electrophoresis with a range of stains, 2D immunoblotting, 2D high-performance liquid chromatography (HPLC), 2D difference gel electrophoresis (DIGE), and increasingly mass…
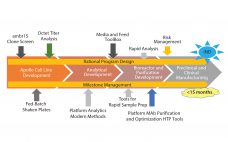
Rapid Development of High-Quality, Robust Mammalian Cell Culture Manufacturing Processes
With increasing industry emphasis on providing both rapid and robust processes, companies are reaping the benefits of new tools for risk management and process analytical controls. As a current example of these approaches, Fujifilm Diosynth scientists have accelerated the development process from gene to finish by shortening the timeline, incorporating quality by design (QbD) principles, and designing the process to be as robust as possible. When the Apollo mammalian expression cell line was introduced three years ago, the time from…
S-Bio Launches EZGlyco mAB Kit with 2-AB for N Glycan Sample Preparation from IgG in cell culture for HPLC analysis
S-BIO1 has launched today EZGlyco™ mAb-N Kit with 2-AB, a breakthrough new product that enables scientists to analyze N -glycans in IgG and Fc-fusion proteins from culture supernatant samples in a rapid, simplified and streamlined single tube operation. The kit is a valuable tool supporting customers in glycoprotein characterization for biotherapeutic development. Therapeutic monoclonal antibodies (IgG, mAb) are routinely expressed in recombinant expression systems. N-glycosylation is one of the most important post-translational modifications. Monitoring of glycosylation in antibody development and…
Benefits to Strategic Outsourcing of Biologics Analytical Studies
Michael Merges (director of Catalent Biologics), BPI Theater @ BIO, June 8, 2016, 1:40–2:00 pm Many small companies do not have the expertise or specialized technologies to do the necessary testing that underlies biologics manufacturing. Expertise is needed in microbiology, raw materials testing, characterization, and method development. At the same time, testing is taking place earlier in product development. Bioassays are the most outsourced service, and that is expected to continue. Catalent has over 25 years of experience in characterizing…