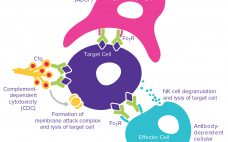
The complexities of biomanufacturing combined with heterogeneity introduced by cellular expression systems present significant challenges to assessing the quality of biologics such as monoclonal antibodies (MAbs). Information related to the critical quality attributes (CQAs) of MAb drug candidates is unknown during early phase drug development. It must be established empirically by physical, structural, and functional analyses as early as possible to accelerate development and mitigate risk through greater understanding of product characteristics. High-resolution analytical techniques are required to answer questions…
Search Results for: antibody characterization
Characterization and Lot Release Assays for Antibody Drug Conjugates
Antibody-drug conjugates (ADCs) add an additional level of challenge to testing of biotherapeutics. Besides the antibody, which needs to be evaluated for potential and known mechanisms of action (MoA), there is a cytostatic compound conjugated that alters the behavior of the antibody-vehicle within the typical assays. Therefore, characterization of new innovators as well as biosimilarity assessment is even more challenging than it is for antibody therapeutics. Using the example of Trastuzumab emtansine, Charles River has set up a panel of…
Uniting Small-Molecule and Biologic Drug Perspectives: Analytical Characterization and Regulatory Considerations for Antibody–Drug Conjugates
Cosponsored by CASSS (an international separation science society) and the US Food and Drug Administration (FDA), the January 2010 CMC Strategy Forum explored antibody–drug conjugates (ADCs), which are monoclonal antibodies (MAbs) coupled to cytotoxic agents. The ADC platform of products is being used more and more for clinical evaluation in oncology. More than a dozen companies are developing several types, including products conjugated with calicheamicin, auristatins, and maytansinoids. Such products use the specificity of a MAb to deliver a cytotoxic…
Antibody and Protein Therapeutic Kinetic Characterization in Bioprocessing
Figure 1. There are a variety of affinity tag-protein strategies, and the use of affinity tags depends on the target protein, the expression system, and the application. The Octet QK and Octet RED systems provide an easy-to-use, label-free, high-throughput system to capture affinity-tagged biologics for quantitative or kinetic biomolecular interaction analysis. Kinetic characterization of biologics during clone selection, screening, and development is critical at all stages of therapeutic engineering and bioprocessing. Key Applications Quantitative kinetic characterization (kobs′ ka′ kd′ KD…
The Critical Role of Predictive Bioreactor Characterization in Pharmaceutical Process-based Upscaling
In bioreactors, microorganisms or cell cultures produce complex therapeutic proteins and other biopharmaceuticals. The industrial production of those active pharmaceutical ingredients usually involves a seed train: the cells are run through many cultivation systems, which become larger with each passage (Upstream Process). An adequate number of cells for the inoculation of large-scale production bioreactors of 10,000 liters or more is generated. A prominent example from the growing mammalian cell culture processing sector is the upstream production process of monoclonal antibodies.…
Antibody-Derivative Biotherapeutics: Fragments and Fusions Define the Future
Monoclonal antibodies (MAbs) remain the dominant biopharmaceutical product class, but as biotechnologies have advanced in recent decades, developers have found ways to exploit their “magic-bullet” capabilities while putting aside their limitations. That has led to a new generation of antibody-related therapeutics created by cutting and pasting molecular domains. Researchers are mixing and matching functional moieties of antibodies and other molecules to create custom-designed proteins with powerful efficacy and tunable targeting. A simple search at Taylor & Francis Online (https://www.tandfonline.com), the…
Expression of Recombinant Antibody Fragments: High-Density Fermentation in Multiuse and Single-Use Systems
Single-use fermentors (SUFs) offer dramatic advantages over traditional stainless-steel clean-in-place (CIP)/sterilization-in-place (SIP) fermentors. Single-use technology eliminates the need for steam, chemicals, and water to clean and sterilize stainless vessels, which provides a direct reduction in capital cost and environmental-impact mitigation. Single-use platforms also increase equipment and process flexibility significantly, making it possible to switch campaigns from one product to another in minimal time, while eliminating cleaning and associated validation steps (1). Both Cytiva and Thermo Fisher Scientific offer SUFs that…
eBook: Antibody–Drug Conjugates —
A New Generation of Approaches Is Changing the Game
Combining large proteins with linkers and cytotoxins, antibody–drug conjugates (ADCs) may be the most complex drug molecules in development today. Despite early promise and product approvals, a number of technical concerns arose during product and process development. Characterizing and ensuring consistency in the number of small molecules that attach to the antibody — as well as ensuring their proper attachment and biophysics — all present significant challenges to ADC developers. Solving early problems associated with product quality has introduced a…
Cell-Line Development: Accelerating Antibody Discovery By Monitoring Titer and Glycosylation with the Octet Platform
Cell line development involves the screening of thousands of clones in an effort to find the few optimal clones that are stable, grow as expected, and produce high yields of the bioproduct. The time it takes from engineering an optimal cell line to the production of the target biologic can be prohibitive and may differ from molecule to molecule. While expression-level analysis like titer screening is carried out early, other critical quality attributes (CQAs) such as glycan characterization are often…
SARS-CoV-2 Hyper-Immunoglobulin: Purification and Characterization from Human Convalescent Plasma
The novel severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) emerged as a major pandemic coronavirus disease in 2019 (COVID-19) and since then has killed many people and paralyzed the global economy (1, 2). With specific antiviral therapeutic agents or antibodies yet to be approved, other antivirals and novel vaccine strategies have been essential to containing the virus and disease transmission. Passive antibody therapy can be used to limit the scope of epidemics by providing patients with antibodies that recognize…