Welcome New Editorial Advisor Kavita Ramalingam Iyer is associate director and product lead of GRACS-CMC for vaccines at Merck Sharp & Dohme Corp. She received her PhD in biotechnology from Anna University (India) and completed a postdoctoral fellowship at the University of Minnesota (focusing on antibody engineering and synthetic biology) before joining Merck in 2008. Kavita has over 10 years of pharmaceutical industry experience leading chemistry, manufacturing, and controls (CMC) development; manufacturing; establishment of good manufacturing practice (GMP) facilities; technology…
Search Results for: regenerative medicine
Assuring Multipotency of human Mesenchymal Stem Cells (hMSC)
Over the past decade, stem cell research has provided new avenues for deeper investigation into tissue repair and aging processes, as well as regenerative medicine methods. One of the major players in such research endeavors are mesenchymal stem cells (MSC), also known as mesenchymal stromal cells. MSC are typically found in bone marrow, adipose, placental, and umbilical cord tissues1 and are a type of adult stem cell. In vivo, these cells are headquartered in special microenvironments or ‚Äúniches‚ÄĚ in the…
Spotlight for January-February
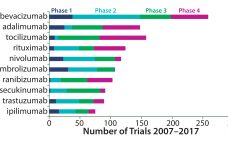
Tracking Antibodies in the Clinic In tracking clinical trial activity around the world, GlobalData has identified 5,273 clinical trials of monoclonal antibodies (MAbs) that started between 1 January 2007 and 31 December 2016. The top three drugs in numbers of trials were bevacizumab, adalimumab, and tocilizumab. Rounding out the top 10 were rituximab, nivolumab, pembrolizumab, ranibizumab, secukinumab, trastuzumab, and ipilimumab. Healthcare analyst Marco Borria said, ‚ÄúAll the top 10 drugs are marketed, and the majority of trials initiated for them…
Single-Use Production Platforms for Biomanufacturing
The pipeline of biopharmaceuticals remains strong, and the market for biologics could exceed US$450 billion by 2025. Analysts predict that sales within segments such as regenerative medicine and antibody‚Äďdrug conjugates (ADCs) will grow faster than 20% each year. Yet considerable challenges remain for biopharmaceutical companies to overcome if they wish to be successful. First, they must reach the market quickly. Analysis by the Boston Consulting Group shows that the proportion of available value that a newly launched product can capture…
Final Thoughts: Single-Use Platforms
The development and launch of new biopharmaceutical products is a very challenging and risky process. Sponsors must get their products into clinical testing as quickly as possible to beat their competition and take the greatest share of the market. Yet that need for speed must not lead to companies launching products with inefficient manufacturing processes that ultimately will leave them vulnerable to attack from low-cost competition. Single-use systems have been a significant enabling technology for companies developing new biologics because…
Advanced Control Strategies at Biotech Week Boston
Attendees at this year‚Äôs Biotech Week Boston (24‚Äď28 September) had the opportunity to participate in several preconference symposia on the first day, including one on advanced control strategies for bioprocessing and biomanufacturing. Chaired by William Whitford (GE Healthcare), the session included presentations from Dan Kopec (Sartorius Stedim Data Analytics), Markus Gershater (Synthace), Jonathan Bones (National Institute for Bioprocessing), Robert Thomas (Loughborough University), Chris McCready (Sartorius Stedim Data Analytics), and Victor Konakovsky (Newcastle University). BPI has collaborated with conference organizer KNect365…
Isolator advantages in Cell Therapy Production
With numerous cellular and gene therapy products seeing strong initial clinical successes, investment in next-generation technologies by both large bio/pharma companies and start-up specialist firms has been significant. In fact more than 750 companies worldwide declare themselves to be in the ‚Äúregenerative medicine‚ÄĚ market space, with a high percentage of year on year increases in the number of cellular and gene therapy drugs in clinical trials. Many of these companies are eager to advance from early stage clinical trials to…
Addressing Knowledge Gaps and Skills Development: Modular Training Keeps the Bioindustry at the Leading Edge
One major challenge facing the global bioindustry today is finding talented individuals to work in the type of highly skilled interdisciplinary environments necessary for effective bioprocess development. Ideally, such individuals require a combination of technical knowledge and expertise spanning biological sciences, physical sciences, mathematics, and engineering. Numerous industry surveys have repeatedly stressed the lack of suitably trained individuals equipped with necessary skills to work at the biology‚ąíengineering interface to meet the growing and changing demands of industry. The challenge is…
PCT Establishes Global Contract Development and Manufacturing Services Platform with Opening of Yokohama, Japan Facility
Hitachi Chemical Co., Ltd. (HCC), a global service provider for the cell therapy industry through its PCT development and manufacturing service platform, today announced the completion of construction and opening of its Yokohama, Japan facility on October 11. This facility, as well as the existing two U.S. PCT facilities, will share the same global PCT service platform for quality and information systems, manufacturing operations, and technology transfer protocols, ensuring a seamless approach to serving clients and accelerating creation of a…
October From the Editor
Often I am dismayed to hear local news reporting of early stage clinical milestones for therapies that may still be years in development. I assume that those reporters and news editors receive pretty much the same press releases that we do here at BPI. But I worry that a more general readership hears only the hype, missing details of the process and time required for approval. More than one letter to the editor has opined that ‚Äúdelays‚ÄĚ in bringing new…