The CASSS chemistry, manufacturing, and controls (CMC) Strategy Forum on 23 January 2019 in Washington, DC, was entitled, “The Development of Patient-Focused Commercial Specifications Through Understanding of Clinical Relevance and Criticality of Quality Attributes.” This forum covered the definition, identification, control, and management of patient-focused attributes throughout the life cycle (from discovery through approval) of biological products, including vaccines. Participants investigated how to differentiate through the product development life cycle which attributes are “clinically meaningful” from those applied for manufacturing…
Search Results for: antibody characterization
Developing Advanced-Therapy Products Through Global CDMOs
Tremendous growth in the cell and gene therapy (CGT) industry is driving unprecedented demand for manufacturing services. To be sure, advanced-therapy developers increasingly are choosing to install in-house capabilities. Doing so can offer companies greater control of their processes, timelines, and budgets than they might have when outsourcing products (1). But industry experts agree that contract development and manufacturing organizations (CDMOs) will remain integral to CGT manufacturing and commercialization (1, 2), especially with veteran contract partners scrambling to acquire CGT…
Toward a Roadmap for Cell-Free Synthesis in Bioprocessing
Cell-free synthesis (CFS), also known as cell-free transcription and translation, supplements cellular components (either a cell lysate or purified recombinant elements) with nucleotides, amino acids, metabolic intermediates, and salts to produce a nucleic acid or protein from a genetic template added to the reaction. This exciting technology has seen a substantial increase in both academic and commercial interest over the past decade (1). Interest stems in large part from the potential to democratize access to the machinery of biology by…
Microbial Expression and Purification: One Company’s Historical Perspective
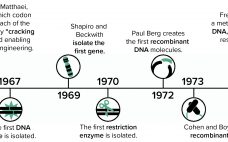
Since the dawn of the recombinant DNA era in the 1970s, New England Biolabs (NEB) has been integrally involved in expressing and purifying proteins, both for its own research interests and for biomanufacturing processes. In 1978, the company began screening microorganisms for restriction enzymes. Our scientists remember the challenges met in purifying limited amounts of restriction enzymes and other proteins from native organisms isolated from the environment. The efforts of those scientists to clone, overexpress, and purify restriction enzymes from…
Ask the Expert: Translating Inhaled and Nasal Technologies for Biologics Delivery
Gastric delivery is unachievable for most biopharmaceuticals, so drug developers formulate biologics primarily for intravenous infusion or injection. However, inhaled and nasal delivery options are attracting considerable attention because they enable targeted delivery of a wide range of therapeutic proteins. On 23 June 2020, Mark Parry (technical director at Intertek) presented an “Ask the Expert” webinar that described critical considerations for inhaled and nasal delivery for biologics. Parry’s Presentation Because biopharmaceuticals are complex products, developers need compelling reasons to choose…
Development of an Advanced Gene Therapy Platform
Steve Pincus, PhD, head of science and innovation, Fujifilm Diosynth Biotechnologies Fujifilm Diosynth Biotechnologies (FDB) is a contract development manufacturing organization (CDMO) with four sites in the United Kingdom, Denmark, and the United States (Research Triangle Park, NC, and College Station, TX). The sites in Denmark, the United Kingdom, and North Carolina specialize in proteins and monoclonal antibodies (MAbs) with capabilities up to 20,000 L. The Texas site works primarily on virus-based therapies and was founded by Texas A&M University…
The Upstream Perspective: Taking Efficiency Beyond Cell-Line Development
With 20 years of experience in the biopharmaceutical industry — at Genentech, Applied Biosystems, Cell Genesys, Cellerant Therapeutics, and Bayer — Yuval Shimoni has written frequently for BioProcess International on a number of production topics. Those have ranged from process improvements and bioreactor scale-down validation, to raw materials management, to addressing variability and virus contamination events. For this featured report, we discussed hardware and instrumentation, quality by design (QbD) and related approaches, and other strategies that can take expediting upstream…
The Downstream Perspective: Putting Product Knowledge to Work Using Technological Innovations
After over a quarter century in the industry — including downstream processing (DSP) and manufacturing directorships at Boehringer Ingelheim and leadership roles in technology development, quality, and manufacturing at Novasep — European consultant Margit Holzer is a recognized expert in downstream processing of biopharmaceutical products. Holding a doctorate in biotechnology from the University of Natural Resources and Applied Life Sciences in Austria, Holzer is familiar to BPI readers as both an author and conference participant (1, 2). And in May…
Get to IND Faster: Accelerated and High-Performance Cell-Line Development
In April 2020, Samsung Biologics hosted a webinar with John Gill, the company’s director of cell-line development (CLD). He focused first on chemistry, manufacturing, and controls (CMC) activities needed for preparing an investigational new drug (IND) application. Then he introduced a fast timeline for managing CLD programs to accelerate client projects to success. CMC Activities Samsung Biologics is a fully integrated contract development and manufacturing organization (CDMO). Starting with a client’s gene of interest (GoI), Gill’s group tailors CLD to…
Viral-Vectored Gene Therapies: Harnessing Their Potential Through Scalable, Reproducible Manufacturing Processes
We might not associate the jazz queen Ella Fitzgerald with 21st-century gene-based therapies, but the First Lady of Song was on to something back in 1939 when she sang “’T’Ain’t What You Do (It’s the Way That You Do It).” Although demonstrating the safety and efficacy of gene-based therapies in rigorous clinical trials is essential for gaining product approval from regulators, doing the bare minimum is insufficient. The way that such products are produced also matters. Manufacturing processes and protocols…