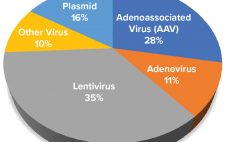
Advanced therapy medicinal products (ATMPs) are engineered to replace defective, disease-causing genes to compensate directly for a genetic defect or to encode a therapeutic protein construct (e.g., chimeric antigen receptor, CAR) for disease treatment. In most instances, a viral vector delivers the engineered genetic payload, targeting cells in situ or ex vivo through cellular modification, expansion, and infusion into a patient. Clinical successes of ATMPs bolstered by regulatory approval of products such as Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), Kymriah (tisagenlecleucel,…
Expression Platforms
eBook: Addressing Production Complexities — Strategies for Working with Difficult and Susceptible Proteins
All proteins are complex — but some are more complex than others, particularly when it comes to recombinant protein expression and production in commercial quantities. What works in a research laboratory to make a milligram of pure protein for study won’t necessarily work on a manufacturing floor to make kilogram batches for drug-product formulation. An increasing number of technological options are available, however, from a simple switch in expression host or adding folding steps in downstream processing to special genetic…
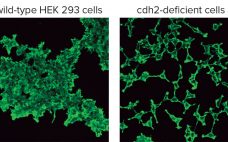
Creating Novel Cell Lines By Genome Editing: Simplifying Cell-Based Assays and Improving Production of Biomolecules
Cultured cell lines have a diverse range of applications. They are used broadly by cell biologists, clinicians, tissue engineers, biotechnology scientists, and bioengineers. The most important uses of cell culture are in the cell-based assays and production of biologically active recombinant proteins. In recent years, genome editing has been used widely to study the structure, function, and localization of endogenous proteins in cultured cells. However, applying the same genome editing techniques to cell lines also could improve the propagation of…
eBook: Expression Systems — Increasing Productivity and Reducing Costs
Biopharmaceutical manufacturers are facing increasing pressures to improve productivity and reduce time to clinic and market. Increasing productivity begins with selecting the appropriate expression system for each protein. Current efforts to boost expression titers also are focused on implementing selection/screening technologies, engineering Chinese hamster ovary (CHO) expression systems, and accelerating timelines for the development of complex next-generation therapies. BioProcess International asked three representatives of the industry’s leading companies to comment on current expression system technologies and strategies Just fill out this…
Improving CHO Cells for Biomanufacturing
Chinese hamster ovary (CHO) cells have been used in biomanufacturing for decades because of their robust capacity to express a range of proteins, such as therapeutic enzymes and monoclonal antibodies (MAbs) at titers measured in multiple grams per liter of culture. Within the available suite of CHO cell lines, the glutamine synthetase knockout (GS-KO) selection system provides industry-leading speed to the identification of high-producing clones for use in biomanufacturing. The GS-KO selection system allows for identification of multiple-gram/L clones in…
Monoclonal Antibodies: Beyond the Platform in Manufacturing
The vast majority of monoclonal antibody (MAb) production processes are based on fed-batch Chinese hamster ovary (CHO) cell culture and protein A affinity column chromatography capture. Increasing cost-consciousness — among innovator companies as well as biosimilar makers — has many companies looking “beyond the platform” for less expensive alternatives that may provide better results. Here the BPI editors review some state-of-the-art alternatives in upstream and downstream MAb drug substance bioprocessing as well as drug-product manufacturing. The current “gold standard” platform…
eBook: The Commercial Expression Systems Market — What Has Changed in the Past Decade
A decade ago, BioPlan Associates prepared the findings of its 2008 directory of expression system technologies that were being promoted or considered likely to be suitable for commercial licensing for biopharmaceutical manufacturing (1). Due in part to the relatively slow advances in this critical area of bioprocessing, this study remains perhaps the only directory of biopharmaceutical-relevant expression systems available for licensing. Here I discuss aspects of related bioprocessing technologies that have and have not changed in the past decade. Expression…
eBook: Production Cell-Line Development and Control of Product Consistency During Cultivation — Myths, Risks, and Best Practices
Health authorities are requesting substantial details from sponsors regarding practices used to generate production cell lines for recombinant DNA–(rDNA) derived biopharmaceuticals. Authorities also are asking for information about the clonality of master cell banks (MCBs) and control strategies to minimize genetic heterogeneity. Such requests are prompted by recent reports indicating “nonclonality” for certain production cell lines. To address these and related issues, the CASSS CMC Strategy Forum on “Production Cell Line Development and Control of Product Consistency During Cell Cultivation:…
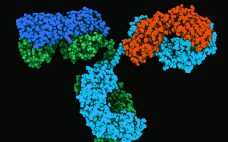
Therapeutic IgG-Like Bispecific Antibodies: Modular Versatility and Manufacturing Challenges, Part 2
Monoclonal antibodies (MAbs) are bivalent and monospecific, with two antigen-binding arms that both recognize the same epitope. Bispecific and multispecific antibodies, collectively referred to herein as bispecific antibodies (bsAbs), can have two or more antigen-binding sites, which are capable of recognizing and binding two or more unique epitopes. Based on their structure, bsAbs can be divided into two broad subgroups: IgG-like bsAbs and non–IgG-like bsAbs. We have chosen to focus on the former in this review. Part one provides a…
An Approach to Generating Better Biosimilars: Considerations in Controlling Glycosylation Variability in Protein Therapeutics
The global market for biotherapeutics has expanded extensively over the past decade and is projected to account for more than a quarter of the pharmaceutical market by 2020, with sales exceeding US$290 billion (1). Continued expansion of the biosimilar marketplace has led to many commercial opportunities and technical challenges. The biological systems used to manufacture such drug products are inherently variable — a feature that has important consequences for the reproducibility, safety, and efficacy of the resulting products. Therefore, a…