The dynamic binding capacity (DBC) of a chromatography resin represents the total amount of target protein that the resin will bind under actual flow conditions before significant breakthrough of unbound protein occurs. This is a useful parameter for predicting what the process performance of a resin will be in actual use. DBC affects the overall amount of resin that can be packed in a given column for a process — and the number of batches that can be processed cost-effectively…
Separation/Purification
Setting a Cornerstone for Platform Purification of Exosomes
Exosomes are a subject of rapidly growing therapeutic interest in the biopharmaceutical industry for two principal reasons. The first reason is that they are the primary communicators of instructions from source cells to target cells. Exosome surface features define their destination. They recognize complementary features on target cells, dock with them, and deliver their programmed instructions in the form of microRNA. The second reason is that exosomes are immunologically silent. As normal human cell products, and by contrast with gene…
Monoclonal Antibody Aggregate Polish and Viral Clearance Using Hydrophobic-Interaction Chromatography
Hydrophobic Interaction chromatography (HIC) is a powerful polishing tool for the downstream purification and manufacture of biotherapeutics. HIC offers orthogonal selectivity for the clearance of difficult process and product-related impurities such as aggregates, host cell proteins and endogenous and adventitious viruses. In this study, a family of POROS HIC resins with novel ethyl and benzyl chemistries was used to successfully polish two clinical stage monoclonal antibodies harboring very high levels of product aggregation (>10%). In addition to aggregate removal, viral…
Adenovirus Downstream Process Intensification: Implementation of a Membrane Adsorber
Historically, companies developing vaccines have used attenuated pathogens, inactivated infectious agents, or antigenic constituents purified from pathogenic sources. In the past 20 years, technological advances such as recombination and viral vectors, have enabled development of vaccines against diseases with previously no available treatments (1). Viral vectors have become one of the most rapidly evolving and promising fields in vaccinology and regenerative medicine. In addition to preventing infectious disease, they have a broad range of potential applications, including treatment of hereditary…
Rapid Mammalian Cell Harvest Without Centrifugation for Antibody Purification: Using a Novel System for Cell Culture Media Clarification
Monoclonal antibody (MAb) expression systems typically use signal peptides to ensure secretion of antibodies into cell culture media. Although that reduces the complexity of purification and prevents the need for cell disruption, it does require using expensive and time-consuming techniques to separate cells from antibody-containing cell culture fluids. In this study, we describe our tests of the novel Sartoclear Dynamics Lab V system (Sartorius S Lab Instruments GmbH and Co. KG) for rapid clarification of cell culture media without requiring…
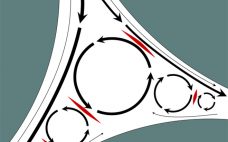
Making Downstream Processing Continuous and Robust: A Virtual Roundtable
Current biomanufacturing is driven to pursue continuous processing for cost reduction and increased productivity, especially for monoclonal antibody (MAb) production and manufacturing. Although many technologies are now available and have been implemented in biodevelopment, implementation for large-scale production is still in its infancy. In a lively roundtable discussion at the BPI West conference in Santa Clara, CA (11 March 2019), participants touched on a number of important issues still to be resolved and technologies that are still in need of…
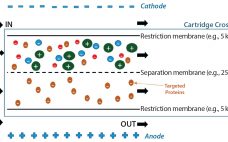
Tangential-Flow Electrophoresis: Investigation of Factors Involved in an Effective Separation of Human Serum Albumin from Human Plasma
Human plasma is a complex mixture of biomolecules such as serum albumin, immunoglobulins, coagulation factors, and others (1). These important protein biomolecules often are present at low levels or lacking in affected patients with certain life-threatening conditions. To extract those valuable proteins, a number of purification methods have been developed over time. Plasma fractionation can be traced back to the middle of the 20th century, when Edwin Cohn of Harvard University developed the first industrial process to purify proteins from…
Intensification of Influenza Virus Purification: From Clarified Harvest to Formulated Product in a Single Shift
Influenza is a global respiratory disease with an estimated mortality of up to a half million people per year (1). The majority of traditional influenza vaccines are still produced in eggs. Downstream processing typically consists of clarification by centrifugation, concentration by ultrafiltration, and purification by ultracentrifugation (2). Recombinant vaccines are most often purified by chromatography. Chromatographic purification of viruses already has achieved major improvements in recovery and scalability (3), but it also is important because it enables virus purification to…
Sticking In or Standing Out? Dichotomy in Vaccine Purification By Chromatography
A general vaccine purification strategy can be divided into three stages, with one or more steps for each stage. The first stage is to concentrate and isolate the target molecule quickly to remove it from conditions that could lead to its inactivation or loss. Intermediate purification seeks to remove remaining contaminants, typically using an orthogonal approach. That is followed by a polishing step in which trace impurities are removed through high-efficiency steps because those impurities usually are similar to the…
Process Analytics and Intermediate Purification of Bispecific Antibodies with a Non-Affinity Platform
The therapeutic benefits of monoclonal antibodies (MAbs) have been demonstrated in recent decades with uncontestable success as treatments for human disease. Despite MAbs’ key features such as specificity, selectivity, and safety, the format has limitations (1, 2). Bispecific antibodies may overcome number of difficulties (3). Multiple formats of bispecific antibodies have been developed, although only the κλ-body is fully human and devoid of linkers or mutations. It requires no genetic modifications of heavy and light chains and results in bispecific antibodies…