For current process development phases, many biomanufacturers’ attention is directed increasingly to the first unit operation in downstream processing, which is the removal of cells and cell debris from culture broth and clarification of supernatant containing a biopharmaceutical product. Given the high cell densities achievable with both mammalian and microbial cell culture processes, primary recovery can be a significant challenge. The current trend in cell culture is to increase product titers with enriched culture media, improved cell productivity, and increased…
Separation/Purification
Membrane-Based Clarification of Polysaccharide Vaccines
Polysaccharide vaccines are essential for protection against infectious diseases, which remain an alarming cause of mortality. The first glycoconjugate vaccine for use in humans — a Haemophilus influenzae type b (Hib) conjugate — was licensed in the United States in 1987. This vaccine successfully reduced the incidence of invasive Hib disease in childhood and led to the further development of conjugate vaccines designed to prevent infection by other encapsulated bacteria (1). Polysaccharides are relatively complex carbohydrates made up of many…
Special Report: A Strategy for Cost-Effective Capture Using Agarose-Based Protein A Resins
It is well recognized that the cost of Protein A resins is substantial. If a developmental monoclonal antibody (MAb) makes it to marketing approval and manufacturing, the high cost of purification using a Protein A resin is amortized over a large number of purification cycles, and the contribution to cost of goods is reduced to acceptable levels. However, a high percentage of clinical projects will fail, and the Protein A resin will be used only for a small number of…
Innovative Downstream Purification Solutions for Viral Vectors: Enabling Platform Approaches to Advance Gene Therapies
Over the past decade, gene therapy applications and their importance in the biopharmaceutical industry have been increasing. Gene therapies promise versatile treatment options that could revolutionize and transform medicine. As treatment modalities, they offer the possibility of long-term and potentially curative benefits to patients with genetic or acquired diseases. Gene therapies are designed to treat disease by delivering genetic material that encodes a protein with a therapeutic effect into a patient’s cells. It can be used to replace a missing…
Viral Clearance in Antibody Purification Using Tentacle Ion Exchangers
Manufacturers strive toward cost-effective purification of target molecules and a high level of confidence that their biologics are safe and not compromised by the presence of endogenous retrovirus-like particles or adventitious viruses (1). Reliable reduction of viral particles throughout downstream purification processes must be ensured through different techniques such as chemical treatment, filtration, and chromatography. Common monoclonal antibody (MAb) purification schemes use both cation- and anion-exchange chromatography steps (CEX, AEX). Although CEX (to remove product- and process-related impurities) is not…
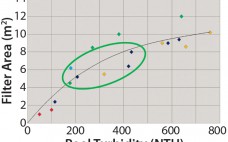
Development of a High-Performance, Integrated, and Disposable Clarification Solution for Continuous Bioprocessing
Current bioprocesses combine fed-batch cell culture with batch-wise downstream processing steps. To achieve integrated upstream and downstream continuous manufacturing, the industry has been in need of a continuous cell separation and clarification solution for bioprocess fluids from bioreactors. The Cadence Acoustic Separator from Pall Life Sciences provides this solution, with continuous first-stage clarification without the need for filter media in a scalable, single-use format with no negative impact on product attributes. The Cadence Acoustic Separator delivers cost and time savings…
Optimization and Scale-Up of HCIC-Based MAb Purification Processes, Part 2
In multistep schemes, hydrophobic charge-induction chromatography (HCIC) has been shown to contribute effectively to clearance of Chinese hamster ovary (CHO) host-cell proteins (CHOPs), DNA, and viruses. When used for capture chromatography, HCIC can provide better aggregate clearance than protein A sorbents can. Chen et al. enhanced clearance of aggregates, CHOPs, and product- related impurities by controlling HCIC based on both pH and the presence of binding-promoting salt in the wash and elution buffers used (1). Taken together with our findings…
An Industrial Platform Solution for Antibody Fragment Purification
Compared with traditional approaches such as chemotherapy and radiotherapy, monoclonal antibodies (MAbs) have become the most successful cancer treatments in the past 20 years (1). With great clinical success in many therapeutic areas, MAbs now account for >40% of the entire biotechnology drug market, and sales are projected to be >US$160 billion over the next few years in the United States alone (2). More than 35 MAbs have been approved for clinical use, and hundreds more are filling industry development…
Optimization and Scale-Up of HCIC-Based MAb Purification Processes, Part 1
Monoclonal antibodies (MAbs) serve important medical needs in cancer treatment as well as that of autoimmune and infectious diseases (1). Antibodies are also widely used in clinical diagnostic assays. They can be coated on solid surfaces to bind specific analytes, conjugated to reporter molecules (either as whole antibodies or fragments) for analyte detection, used in sensitivity panels for lot-release testing, and supplied as positive controls in diagnostic kits (2). Our study evaluates the use of hydrophobic charge-induction chromatography (HCIC) for…
Upstream Efficiencies, Economic Forces, and Changing Technologies Complicate Separation and Purification
When it comes to biotherapeutics manufacturing, downstream processing groups tend to get “dumped on.” Advances in cell lines, bioreactors, and culture media formulations have increased production output, providing both higher expression titers and greater volumes, but the filters and chromatography columns on the downstream side haven’t kept pace. These century-old technologies haven’t evolved as much and are reaching their limits. Regulatory agencies have contributed to innovation stagnation because they are cautious about manufacturing process changes for fear of undermining quality…