Purification of recombinant proteins remains a critical challenge for applications ranging from basic biological research to the development and production of lifesaving biopharmaceuticals. At laboratory scales, the rapid purification of large numbers of new and uncharacterized protein targets effectively compels the use of affinity tags, which enable reliable purification using simple, established protocols with minimal optimization. Tags cannot be used for therapeutic applications, however, because of their potential for immunogenicity. Thus, for proteins other than monoclonal antibodies (which typically use…
Separation/Purification
eBook: Process-Related Impurities — Emerging Strategies for Detection, Identification, and Management of Host-Cell Proteins
Host-cell proteins (HCPs) represent a major class of process-related impurities (PRIs) that are generated during biopharmaceutical manufacture. Although the vast majority of such proteins are removed from a drug substance during downstream purification, residual HCPs can remain in a finished drug product. Even in minimal concentrations, copurifying HCPs can pose safety risks and compromise protein-product yield, efficacy, and stability. Thus, regulatory agencies consider the presence of HCPs to be a critical quality attribute (CQA). Sufficient clearance of these impurities helps…
Ligand-Based Exosome Affinity Purification: A Scalable Solution to Extracellular Vesicle Downstream Bottlenecks
Novel therapeutics based on extracellular vesicles (EVs) recently passed a critical development milestone. During 2020, some of the first experimental EV products developed by biopharmaceutical companies entered human clinical trials (1–3). EVs are nanometer-sized, lipid-wrapped spheres released by almost every cell type in the human body. EVs are loaded with a cargo of proteins, lipids, and RNA, and they are tagged with surface markers that favor uptake by target cells. Thus, EVs are a key mode of cell-to-cell communication (4).…
Eliminating the Analytical Bottleneck in Production and Purification of mRNA
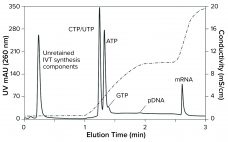
COVID-19 has focused a spotlight on the ability of mRNA technology to accelerate vaccine development and approval (1). That same technology can hasten development and approval of other therapeutic classes, including cancer immunotherapy, protein replacement, and gene therapy. Fulfilling those opportunities imposes significant challenges on process developers and manufacturers to improve existing processes. Scale-up to produce millions of doses (tens of kilograms) compounds those challenges. Furthermore, every step of the journey requires high-performance analytical methods, to ensure patient safety and…
Ask the Expert: Selecting the Right Buffer Management Strategy
Although buffers are among the simplest materials used in bioprocessing, they are critical to biopharmaceutical manufacturing success. Buffer preparation, storage, and handling can require significant investments in time, labor, equipment, and facility space. Jenny Dunker, MSc, and Alexander Troken, PhD (global product managers for customized bioprocess solutions and for process liquids and buffers, respectively, at Cytiva), delivered an “Ask the Expert” presentation on 30 March 2021 to explore strategies for intensifying buffer management. Available Options Biopharmaceutical manufacturers often prepare buffers…
eBook: Chromatography Resins — Addressing Challenges in Biologic Purification Workflows
Although chromatography remains the backbone of downstream workflows, selecting appropriate technologies to optimize processes can be challenging. Numerous resin options are available, and the fact that most biologics are large, complex, and inherently unstable further complicates development of a robust workflow. Because chromatography processes can alter a biologic in ways that could impair its intended therapeutic function, investment in process development is critical. Techniques such as design of experiments (DoE) can be used to identify the best approach during process…
eBook: Buffers — Navigating New Demands on Downstream Raw Materials
Bioreactor titers for monoclonal antibody (MAb) processes have increased significantly since the dawn of the biopharmaceutical industry, yet such gains have instigated bottlenecks for critical high-volume raw materials used in downstream processing, such as buffer solutions. As downstream purification is required for most, if not all, biopharmaceutical products, buffers and their preparation are topics that concern nearly every drug company. But those topics rarely receive direct attention. This BPI eBook explores what factors prompted the current buffer bottleneck and what…
How to Improve the Capturing of Antibody Fragments
Some of the latest promising biopharmaceutical drug substances are antibody fragments. Antibody fragments are either separate functional subunits of antibodies or recombinant molecules, which, just like antibodies, are composed of immunoglobulin domains. These drugs offer several therapeutic advantages over conventional monoclonal antibodies. Upstream processing for antibody fragments is easier than it is for standard antibodies. Recombinant-based antibody fragments can be modified to meet specific needs of affinity, avidity, valence, and action mode. They also can be produced in prokaryotic cells…
Ask the Expert: FPLC Column Selection Considerations
On 10 November 2020, BPI presented an “Ask the Expert” webinar with Dan Yukon (head of North American and global SNAP product sales at Astrea Bioseparations) on considerations for selecting analytical fast-protein liquid chromatography (FPLC) columns. With many options on the market, deciding which type and brand to use can be difficult. To help take out the guesswork, Yukon addressed a number of topics, including pressure and volume considerations; column configuration; materials of construction; frit type, design porosity, and mounting;…
Ask the Expert: Centrifugation Guided By Optical Sensors Enables Efficient, Reagent-Free Cell Separation
Ben Josey, PhD (field application scientist at Corning Life Sciences), joined BPI on 3 December 2020 to deliver an “Ask the Expert” presentation about using optical-sensor–guided centrifugation for cell therapy development. Cell-separation techniques fall into four basic categories: filtration, centrifugation, affinity purification, and emerging methods such as microfluidics and acoustofluidics. Selecting the technology best suited to an application requires careful balancing of method precision and process efficiency, the latter of which includes factors such as batch size, time, labor, and…