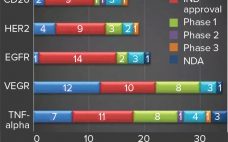
Since the 1986 approval of the first recombinant therapeutic antibody, OKT3, biopharmaceuticals have become a large percentage of overall pharmaceutical company revenue. In 2018, sales of the top five selling recombinant proteins — Humira (adalimumab, AbbVie), Keytruda (pembrolizumab, Merck), Herceptin (trastuzumab, Genentech), Enbrel (etanercept, Amgen), and Avastin (bevacizumab, Genentech), all antibodies — totaled over US$48 billion. The compound annual growth rate (CAGR) for antibodies revenue was about 20% from 2004 to 2014. Those products include naked monoclonal antibodies (MAbs), Fc-fusion…
Economics
eBook: Speed to IND — Balancing Risk and Reward
With so many biopharmaceuticals obtaining breakthrough or fast-track designations, companies that use accelerated strategies to be first in human studies can be left with significant quality and manufacturing challenges that must be solved later on. Despite regulatory encouragement to create solid design spaces and define parameters according to quality by design (QbD), those may go by the wayside given the pressures of speed. The reward is the investigational new drug (IND) application itself — but if companies lock in subpar…
Streamlining the Path to Clinical Manufacturing
Careful consideration of lot size is crucial for multiyear success of a cell therapy business. RoosterBio’s product design and acceleration business works with the company’s customers to help make plans that are appropriate for their stages of product and clinical development. We use the following major considerations in creating a sound multiyear strategy with intermediate milestones: Understand what the future looks like and work backward Use reasonable to conservative assumptions to estimate a range of needs Invest in the right…
Biopharmaceutical Growth in China: Bioprocessing Challenges Are Creating CMO Opportunities
In the past decade, the world has seen rapid growth of more than 15% in China’s biopharmaceutical market (1). According to the second edition of BioPlan’s Advances in Biopharmaceutical Technology in China, the most populous country in the world — with the largest patient groups — has a growing economy with its GDP second only to that of the United States. Rapid urbanization and greater access to national healthcare insurance puts China in second place after the United States for…
Toward Standardized Sample Collections: UK Government Seeks to Expand Cell and Gene Therapy Use
The SAMPLE program — a Standard Approach to ATMP Tissue collection — is intended to help build capacity for the UK National Health System (NHS) to expand the use of next-generation cell and gene therapies for both cancer and noncancer illnesses. Now it has been given the green light by Innovate UK (IUK), the United Kingdom’s technology strategy agency. An award through the Industrial Strategy Challenge Fund is supporting the program for standardizing how cell and gene samples are collected…
eBook: Vaccines – Industry Collaborations to Increase Uptake
Vaccines save millions of lives every year, and the continuing increases in the number of administered doses and worldwide distribution of vaccines for long-standing diseases such as polio and malaria have contributed to the improvement in public health. Vaccine developers and manufacturers are partnering with private and government agencies to raise global vaccine uptake by addressing remaining challenges with production capacity, distribution, safety, and accessibility. And the implementation of new technologies such as virus-like particles and cell/DNA-based vaccines are helping…
Why Conducting Marketing Due Diligence Early in Product Development Is Important
To be successful, a company needs two main ingredients: good science and good business/ marketing. Without good science, a product won’t work, and without a good marketing strategy, a product won’t sell. Two important questions should be addressed: When should marketing groups be involved in product development, and how important is that? The answer to the first is as early as basic research. Why? After a product is launched, a biomanufacturer doesn’t want to discover that its product applies only…
Breaking Through the Noise: An Approach to Differentiating Your Business
When multiple businesses sell the same type of item, why do customers buy from one supplier rather than another? How are vendors able to break through the “white noise” of an industry to stand apart and get noticed? The current bioprocessing and cell therapy vendor markets, about 50,000 vendors are selling to biomanufacturers, universities, and research institutions (www.bioz.com). How do those suppliers get noticed? Market-leading suppliers have established brands that allow them to cross-sell, up-sell, and engage in deep selling.…
Funding Pediatric Cancer Drug Development
Why is it so hard to develop drugs for children with cancer? And what can be done about it? Those questions are central to a Massachusetts Institute of Technology (MIT) study published in JAMA Oncology exploring new business models for funding drug development to treat pediatric cancers (1). Led by Andrew W. Lo (the Charles E. and Susan T. Harris professor at and director of MIT’s Laboratory for Financial Engineering) finds that a collaborative investment structure involving money from private-sector,…
Antibody Derivatives: Deconstructing MAbs for the Next Wave of Biotherapies
Although they make up the largest and most successful category of biopharmaceuticals so far, monoclonal antibodies (MAbs) suffer from certain disadvantages. Some companies are addressing those limitations by deconstructing MAb molecules to create new emergent therapeutics. These antibody derivatives include: antibody fusions and fragments, bispecifics, trifunctional antibodies, and more. This eBook combines market analysis from consultant David Orchard-Webb with technical discussion from BPI cofounder and senior technical editor Cheryl Scott. It also includes commentary from editorial advisor Michiel Ultee and…