In the early 2000s, the trade press was abuzz about an imminent “capacity crunch” in mammalian cell culture. Dire predictions of shortages were based on biopharmaceutical successes to that point, on bursting development pipelines, and on the lengthy timelines and high costs of assembling tens of thousands of liters of stainless-steel bioreactors and supporting infrastructure. Those predictions failed to anticipate several positive developments that would render doom-and-gloom scenarios moot. Notably, yearly improvements in protein titers for MAb processes already were…
Economics
Rapid Deployment of Manufacturing Options: An Analysis of Risks and Benefits
Biomanufacturers seeking the best approach to rapid implementation of flexible manufacturing capacity take into account the benefits presented by different modular construction options. We analyzed different approaches to building manufacturing capacity and assessed the economic benefits of each approach. Our evaluation was based on biopharmaceutical products for which there is an immediate unmet need, such as treatments or vaccinations for COVID-19. Such products also might entail a sudden increase in demand (e.g., expansion of a product indication or sales ramp…
Benefits of Single-Use Standardization: Adopting a Standard Design Approach
It is widely accepted that standardization of single-use designs and assemblies would be beneficial to the biopharmaceutical industry, providing it quickly with simple and economical solutions. Meanwhile, as implementation of single-use technology increases across the biopharmaceutical industry, suppliers are struggling to keep up with demand. That has been evident particularly in current supply issues caused by the COVID-19 pandemic. A widely adopted single-use standardization approach could help alleviate such supply issues. That would not only benefit the industry by helping…
Untapped Potential of Tissue Engineering: The Three Obstacles Holding It Back
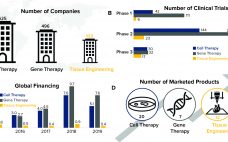
Regenerative medicine is the interdisciplinary field comprising tissue engineering, cell therapy, and gene therapy. These biopharmaceutical modalities, also referred to as advanced therapies, are growing rapidly, characterized by groundbreaking therapeutic advances that have the potential to change how healthcare providers deliver care. As Figure 1 shows, cell and gene therapies have gained traction over the past decade, as evidenced by large increases in investment and the number of marketed products. By contrast, tissue engineering investment and product commercialization has lagged…
The Difficulties of Manufacturing Cell and Gene Therapies At Scale
From large-scale manufacturing of one-size-fits-all blockbusters to small-scale processing of personalized therapies, the biopharmaceutical industry has undergone a revolution over the past decade. Among the standout milestones is the development of advanced therapy medicinal products (ATMPs). More than 1,000 of these research-intensive therapies are progressing through clinical trials toward potential commercial manufacturing. Cell and gene therapies (autologous and allogeneic) are targeted for many incurable diseases and conditions, including autoimmune disorders and cancers. Despite the excitement about ATMP potential, developers and…
Embracing Innovation in Biomanufacturing
Innovations in bioproduction of therapeutics over the past 20 years have led to impressive improvements in product yield, process controls, and manufacturing safety. Industry 4.0 concepts have been embraced across the bioprocess industry and are leading to better bioprocess control through process automation, “big data” and data analysis, process simulations, the industrial internet of things (IIoT), cybersecurity, the cloud, blockchain/serialization, and additive manufacturing. Such advances help to ensure that a process results in the same outcome every time. As Sean…
COVID-19 As a Catalyst for Changing Orphan-Drug Regulations
A long-awaited (and for months withheld) evaluation of the European Union’s Orphan Drug Regulation (ODR) finally reached the interested public in August 2020 (1). Its publication during the public consultation of the European Commission’s “Pharmaceutical Strategy” was well planned because the latter discusses policies on access, availability, and affordability of new medicines. However, the ODR evaluation shows not only that the intentions behind the legislation have not been fulfilled, but also that its generous incentives (extension of market exclusivity and…
eBook: Vaccines — COVID-19 Invigorates a Stagnant Industry
The SARS-CoV-2 novel coronavirus has galvanized what was a stagnant and oligopoly-run vaccine industry. In this inaugural BioProcess Insider eBook, the first of four to be published in 2021, founding editor Dan Stanton explores economic and technical conditions that until now have hampered innovation in vaccine development and discouraged market entry for emerging biotechnology companies. Leveraging commentary from vaccine industry experts and analyzing the range of emerging vaccine modalities, Stanton surveys how industry responses to the COVID-19 pandemic are fostering…
The Green Imperative: Part Two — Engineering for Sustainability in Single-Use Technologies
In BPI’s June 2020 issue, the first installment of this series introduces the study and implementation of single-use (SU) technology to provide a more sustainable manufacturing environment (1). We presented evidence showing that the economic and social benefits of SU systems currently outweigh the residual environmental risks. Not only is SU technology often a better environmental choice than traditional biomanufacturing options, it also is sometimes the only choice for rapid process design and facility start-up. In situations such as the…
Top Trends in Biomanufacturing
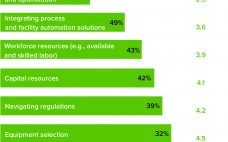
Facing an ongoing pandemic, growing pipelines, and a possible capacity crunch, the bioprocess industry is striving to balance its priorities. Those are some of the key issues to watch according to the 17th annual report and survey of biopharmaceutical manufacturing capacity and production from BioPlan Associates. It includes survey responses from 130 decision-makers (from 33 countries) at both bioprocessing organizations and contract manufacturing organizations (CMOs) and responses from 150 bioprocess industry suppliers (1). Top trends from this report are highlighted…