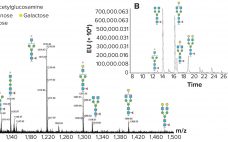
Biopharmaceutical companies are racing to develop vaccines that mitigate the COVID-19 pandemic, taking a wide range of vaccine-development approaches that include traditional modalities and cutting-edge technologies based on DNA and RNA. Vaccine developers are leveraging robust manufacturing concepts and integrated processes to shorten timelines. Advanced analytics also are playing a critical role in ensuring the safety and efficacy of those emerging vaccines. A New Wave of Vaccines Vaccines based on attenuated viruses entail development timelines ranging from four years to…
Product Characterization
Ultracentrifugation for Recombinant Adenoassociated Virus Therapies
Quantitation of different capsid species in viral-vector gene-delivery drugs, including recombinant adenoassociated virus (AAV) therapies, is essential for proper assessment of critical quality attributes before regulatory approval and during commercial production. If rAAV full-capsid percentages are maximized, and variants such as empty or partially-filled capsids are reduced as much as possible, then potency is increased for improved dosing and tolerance outcomes. Traditionally, characterization of AAV purity with respect to empty, full, and other variants has been performed by techniques such…
Development of Patient-Focused Commercial Specifications: Understanding Clinical Relevance and Criticality of Quality Attributes
The CASSS chemistry, manufacturing, and controls (CMC) Strategy Forum on 23 January 2019 in Washington, DC, was entitled, “The Development of Patient-Focused Commercial Specifications Through Understanding of Clinical Relevance and Criticality of Quality Attributes.” This forum covered the definition, identification, control, and management of patient-focused attributes throughout the life cycle (from discovery through approval) of biological products, including vaccines. Participants investigated how to differentiate through the product development life cycle which attributes are “clinically meaningful” from those applied for manufacturing…
Ask the Expert: An Innovative System for Advanced, High-Throughput Aggregate Analysis
Distinguishing aggregated active pharmaceutical ingredients (APIs) from other particle types is critical to evaluating a protein product’s stability, but standard characterization tools struggle to discriminate proteins from nonproteins with similar sizes, shapes, and morphologies. In a 25 June 2020 “Ask the Expert” webcast, Bernardo Cordovez (founder and chief scientific officer of Halo Labs) introduced his company’s Aura subvisible-particle analyzer. He explained how the device combines an innovative microscopy technique with sophisticated imaging software to characterize subvisible particles more quickly and…
Early Stage Analytical Considerations for Late Stage Biologics Success
This BPI Special Report will cover phase appropriate analytical strategies that will balance various drivers to meet overall development goals without sacrificing the requirements of a program. The goal is to provide important recommendations for analytical method development that can help the biopharmaceutical industry identify opportunities to improve lead time and reduce development costs while maintaining required quality standards. Fill out the form below to learn more about analytical method development now. Arugadoss Devakumar, PhD, is director of analytical…
Are You Missing the Bigger Picture with Your AAV Analytics? Fast, Low-Volume Subvisible Particle Analysis for Characterizing Viral Vectors
Subvisible particle (SVP) analysis is a key indicator of stability and safety and is an essential parenteral drug quality metric. Yet assessment of SVPs is especially challenging in adenoassociated virus (AAV) vector formulations, in which limited precious sample is available. Traditional SVP methods consume large sample volumes, while dynamic light scattering (DLS), size-exclusion chromatography (SEC), and visual inspection can miss aggregates in the subvisible range entirely. This special report introduces backgrounded membrane imaging (BMI) technology as a solution for detection…
eBook: Development of Bioassays — A Report from the BEBPA’s First Virtual Meeting
Bioassay development is one of the most challenging aspects of biotherapeutic development. These tests are vital to providing an accurate picture of potency, stability, and biological activity. But bioassay development can be complex and expensive — and often test results can vary. Cell-based bioassays especially lack robustness. In March 2020, the Biopharmaceutical Emerging Best Practices Association (BEBPA) presented its annual bioassay conference in virtual format. Here, BPI’s senior technical editor reports on the conference’s discussions. Read on to learn more…
Building Orthogonality into Biosimilar Testing
Both the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) have developed regulatory guidelines on biosimilars (1, 2). These comprehensive documents provide clear guidance on what the agencies expect from structural characterization studies. Using state-of- the-art instrumentation and techniques is expected, but the use of orthogonal techniques in structural comparability assessments is also required. The application of orthogonal analytical techniques will provide a firm structural foundation to claims of biosimilarity. Characterization methods verifying and supporting conclusions drawn…
Biopharmaceutical Product Specification Limits and Autocorrelated Data
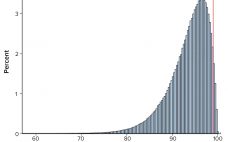
Calculations, including statistical tolerance intervals, can assist in the development and revision of specification acceptance criteria. Manufacturing results for attributes of a biopharmaceutical product can be positively autocorrelated. The sample standard deviation — calculated from limited, positively autocorrelated data — tends to underestimate the long-term process standard deviation (1). In this article, simulated data are used to assess the relative performance of statistical tolerance intervals, intervals calculated using the minimum process performance index Ppk approach, and the sample range. Prevalence…
Measure Twice, Treat Once: Navigating the Regulatory Landscape of Assay Development to Ensure High-Quality CGT Products
Cell and gene therapies (CGTs) are a novel and fast-growing class of transformative therapies designed to address gaps in traditional treatment strategies of some of the most severe diseases. By definition, gene therapy “seeks to modify or manipulate expression of a gene to alter the biological properties of living cells for therapeutic use” (1). That can be either an in vivo delivery of a gene or delivery of a gene to a patient’s cells that are manipulated outside of the…