Regulations require that biomanufacturers assess the intactness of protein and glycoprotein products as well as confirm the terminal sequences to look for existing variations. ICH Q6B guideline section 6.1.1 c states: Terminal amino acid analysis is performed to identify the nature and homogeneity of the amino- and carboxy-terminal amino acids. If the desired product is found to be heterogeneous with respect to the terminal amino acids, the relative amounts of the variant forms should be determined using an appropriate analytical…
Product Characterization
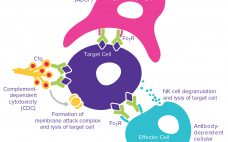
Mitigate Risk with Effector Function Characterization for Antibody Therapeutics
The complexities of biomanufacturing combined with heterogeneity introduced by cellular expression systems present significant challenges to assessing the quality of biologics such as monoclonal antibodies (MAbs). Information related to the critical quality attributes (CQAs) of MAb drug candidates is unknown during early phase drug development. It must be established empirically by physical, structural, and functional analyses as early as possible to accelerate development and mitigate risk through greater understanding of product characteristics. High-resolution analytical techniques are required to answer questions…
Special Report: Current Analytical Approaches to Biophysical Characterization in a Regulatory Environment
Structural integrity of protein-based therapeutics is one of the major challenges in the biopharmaceutical industry. Multiple factors such as product stability, efficacy, and shelf life could be affected following minor changes in manufacturing process. Multiple biophysical methods employing spectroscopic and calorimetric tools can be used to analyse Higher Order Structure (HOS). Moreover, with an increasing demand for generating as much structural information as possible for regulatory submissions, a requirement for these analyses in a GMP environment is also important. This…
Biosimilarity Assessments: The Totality of Evidence Framework
Biosimilars are evaluated through comparisons with their reference products using abbreviated pathways that have evolved significantly over the past few years. Scientists and regulators now accept that some quality attributes can vary from batch to batch over a product’s lifecycle, even for reference products. Moreover, reference and similar biotechnology products can show differences in noncritical quality attributes but still demonstrate comparable efficacy and safety (1). Here we describe a similarity assessment approach that is also applicable to comparability of lifecycle…
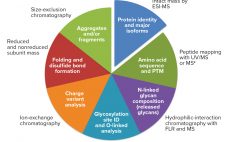
Biopharmaceutical Characterization, Part 2: Applications and Strategies for Diverse Products — A Conference Report
Last fall, KNect365 brought together more than 250 analytical specialists to discuss biological assays and characterization of well-characterized biologics in Rockville, MD. Speakers from the US Food and Drug Administration joined experts from leading biopharmaceutical companies, service providers, and consultancies for case studies, regulatory interactions, sharing perspectives, and learning about emerging technologies. Part 1 of this report in January 2019 focused on the bioassay section of the meeting. Here in Part 2 sponsored by Sartorius, BPI’s senior technical editor reports…
Analytical Challenges: Characterization of Oligonucleotide Therapeutics
Recent approvals of oligonucleotide therapeutics are a clear signal for optimism for this product class. This is supported by the strength of the current pipeline which has over 180 active oligonucleotide clinical programs in various phases of development. Improvements in analytical technology and know-how have played a key role in enabling suitable characterization and quality control strategies to overcome the difficulties associated with testing these complex molecules. Despite the lack of dedicated regulatory guidelines related to characterization or quality control,…
Analytical Tools to Improve Decision-Making During Product Development
Speed to clinic testing — and then speed to market — are highly significant metrics for companies developing biopharmaceuticals. By increasing the pace of drug development, these companies can reduce costs, obtain revenues early, and establish commanding positions in the market relative to their competitors. High-throughput development tools have contributed much to the acceleration of drug development in recent years. Such technologies enable the testing of many process parameters in parallel. Combining them with multifactorial “design of experiment” (DoE) analysis…
Biosimilars: Challenging the Justifications for Clinical Testing
The Biologics Price Competition and Innovation Act (BPIA) of 2009, describes the need for clinical trials as follows (1): “(cc) a clinical study or studies (including the assessment of immunogenicity and pharmacokinetics or pharmacodynamics) that are sufficient to demonstrate safety, purity, and potency in one or more appropriate conditions of use for which the reference product is licensed and intended to be used and for which licensure is sought for the biological product.” However, all the above studies are left…
Speeding Characterization of Biologics: Replace Traditional Assay Technologies with Label-Free Quantification and Kinetics
FortĂ©Bio’s Octet instruments are an ideal replacement for ELISA, HPLC, and SPR techniques in quantification of antibodies and recombinant proteins and in testing product potency for lot release. Bio-Layer Interferometry (BLI) technology monitors biomolecular interactions in real time to determine affinity, kinetics, and concentration. The plate-based, microfluidics-free format offers users several distinct advantages over other technologies. BLI-based systems can achieve higher throughput, with the flexibility to measure two to 96 samples simultaneously. Lower maintenance requirements and increased ease-of-use further shorten…
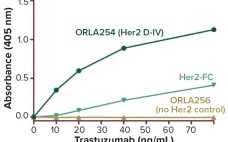
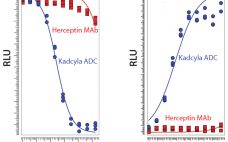
Biological Stealth Bombers: Potency, Regulatory, and Bioprocessing Concerns of Antibody–Drug Conjugates
Seven years ago, the US Food and Drug Administration (FDA) approved the first product in a new class of biologics: antibody–drug conjugates (ADCs). The idea for these products already had been hatched a decade earlier when the promising field of antibody research — touting such molecules as “magic bullets” — had faltered, specifically against oncology-related indications. The early crop of anticancer monoclonal antibodies (MAbs) proved to have only limited efficacy, and interest in developing antibodies as therapeutic agents against cancer…