
A process operator prepares connecting hose for later use in a production process at the Novartis biomanufacturing facility for innovative cell and gene therapies in Stein, Switzerland (https://www.novartis.com)
As editors, we are fortunate to have the opportunity to listen to biopharmaceutical developers and innovators discuss the intricacies of their work. Typically, we find that the best discussions come from asking two fundamental questions: What need did you observe in the industry that drove your work, and what technologies would help you do your job better? Over the past five years or so, the answers have shifted. Cell and gene therapy (CGT) innovators are focusing on increasingly complex diseases and indications. The preference for off-the-shelf allogeneic products is still present, but developers also are not shying away from providing autologous products exclusively.
Although most CGT companies have established processes, they still depend heavily on manual operations and single-source supply chains. So the answers to our second question have changed from “technologies that help me to commercialize my product” to “technologies that make my processes better and faster.” As the number of cell therapy clinical trials increases, such technologies will need to be automated and scalable (see Figure 1 in Gerlovin et al., also part of this month’s featured report). Equally important, manufacturers will need better control of their processes and better assurance of their supply chains.
The discussions below and in the following articles focus on key areas in need of improvement and the obstacles that cell therapy manufacturers are facing as a result of rapid growth, the COVID-19 pandemic, and lack of standardizations. Below, experts from two 2020 industry conferences highlight the cell therapy industry’s concerns and difficulties, and representatives from two innovation companies provide overviews of how their technologies aim to address some important needs.
Essential Steps for Moving Forward
In May 2020, the International Society for Cell and Gene Therapy (ISCT) held its annual meeting in Paris, France. At that time, many countries had mandated lockdowns and other restrictions that had effects on several industries. After the conference, we spoke with Miguel Forte, ISCT’s former chief commercialization officer and head of its commercialization committee, to gain his insights on CGT supply chains and patient access to advanced-drug products.
How stable are the supply chains that support CGT research, development, and manufacturing? Supply chain elements have evolved significantly since I first was involved in such logistics. That was when the CGT industry was moving fresh autologous products, which obviously was challenging timewise. Now, many products are allogeneic or autologous (but cryopreserved). Consequently, the supply chain buffer has become much more significant. Nevertheless, it is important to ensure strict adherence to chain-of-custody protocols. The timeframe in which dry shippers can transport cells also remains limited — about a week, sometimes more. So delivering CGT products to patients remains a big challenge. Strategic decisions also need to be made about how to prepare such products for transport, which raises questions about where companies should perform manufacturing: What’s my geographic spread? How do I test a product in clinical trials and subsequently be ready to deploy it at a large scale when I get to the market?
All of those concerns are relevant, and COVID-19 has diminished patient access to hospitals — and thus to clinical trials. Regulatory authorities have issued guidance on how to manage studies during this pandemic, yet many trials have been interrupted and only now are reinitiating. At my company, Bone Therapeutics, we were about to start a study when the pandemic began, and we needed to wait for conditions to improve or for appropriate safety protocols to be developed. Now we’re seeing active recruitment, with patients able to access hospitals for treatment and candidate products being shipped under certain conditions. The return to clinical development is important because it enables us to get candidate therapies to patients and generate data needed to advance those therapies toward commercialization.
Some conference presenters remarked that the CGT industry has reached a critical inflection point in its commercial-scale manufacturing. How ready is the industry for such scales? The industry is approaching readiness. Its maturation requires improvements in manufacturing, short-term storage, and supply chains to help more products reach more patients. The CGT industry also must be able to establish a drug product’s true value and address the unwillingness of some healthcare insurers to pay for emerging products. All of those factors are evolving significantly.
Many new solutions are facilitating the technical aspects of CGT manufacturing. Consequently, CGT developers are getting prepared to scale up. Increased use of automation also will reduce costs and improve batch-to-batch consistency. And from all those developments, we find that standardization is coming into place. Developers have gotten to a point where they are comfortable with certain blocks of the manufacturing process and no longer need to tinker with them. Such standardization is a clear signal of the CGT field’s increasing maturity. The industry also has begun to engage healthcare companies in discussions about different approaches to pricing and payments to increase patient access to CGT products.
Much still needs to be done, and the work to undertake often differs by product. The CGT field is reaching an impending maturity stage. It can respond to the challenges that I mentioned, and it’s able to get products to patients. But quite a lot remains to be done along the chain of value, all the way from preclinical through clinical, regulatory, and commercial manufacturing activities.
Some speakers highlighted the complexity of current business and pricing models. What new models are emerging, and how might those ultimately improve patient access to CGTs? CGT costs relate primarily to research and development (R&D), production, and distribution. The other side of pricing involves willingness to pay — that is, how much patients will (or can) pay and how much healthcare insurers and providers are willing to cover for a given therapeutic benefit. The bigger the benefit and the bigger the need, the more money that patients might be willing to pay.
Consider autologous products, for which you must create one batch per patient. Such a model creates a significant manufacturing burden to treat only one patient, and that generates significant costs. Nevertheless, some such therapies address unmet medical needs in significant ways — such as by reducing mortality rates among patients with certain cancers and helping patients manage enzyme defects. Such therapies carry considerable value. If there is an opportunity to treat or even cure a disease with an innovator autologous therapy, then that could increase willingness to pay high drug prices. On the other hand, if a therapy is designed to treat conditions that are not as dire as cancer or if it diminishes a disease’s morbidity rather than curing it, willingness to pay will decrease to reflect that different context.
That said, high costs for autologous products highlight the value of allogeneic “off-the-shelf” CGTs. Materials for such therapies yield multiple doses. Thus, allogeneic therapies can be manufactured at significantly lower cost, enabling companies to pass lower-priced therapies to more patients than they could with autologous products.
I can speak to these business conditions because I’ve helped to develop autologous T-cell–based oncology products within the model of high cost, high value, and high willingness to pay, and I am working now with an allogeneic therapy for patients with bone regeneration needs — conditions that often (but not necessarily) are associated with mortality or significant morbidity. Because that therapy is allogeneic, we can treat large numbers of patients with unmet medical needs using an off-the-shelf product. That also enables us to bring the cost of gene therapy production closer to that for traditional biologics manufacturing. Consequently, we are creating a business model that makes more sense for payers and patients and that better corresponds with willingness to pay. Such diversity of offerings is another signal of the CGT field’s maturity.
Growth Adds Opportunities and Difficulties
About five months after the ISCT conference, different speaker presentations and panels at the Cell and Gene Bioprocessing and Commercialization conference (October 2020) echoed Forte’s assessment of the CGT industry. He led a panel titled “COVID-19: Trailblazers in the CGT Industry.” Participants included Claudia Berron (Avantor), Chris Gemmit (Sentien Biotechnologies), John Lewis (Aegis Life Inc. and Entos Pharmacetuticals), Racheli Ofir (Pluristem Therapeutics), Lawrence Thompson (Pfizer), and Camilo Ricordi (National Academy of Inventors, Italian Supreme Council of Health).
One discussion point centered on the obstacles that manufacturers are facing. CGT developers need ways to streamline R&D, production, and commercialization while optimizing processes and keeping them on time. Ricordi noted that scaling up from hundreds of doses to thousands and moving from two-dimensional systems to three-dimensional bioreactors are common challenges. Panelists also discussed the need for obtaining enough raw and starting materials to expand cells to the levels needed for future CGTs. They agreed that such “challenges” came with the opportunities and the successes of a rapidly growing field and the need to generate high volumes.
Lockdowns and other pandemic restrictions had hindered some CGT development and manufacturing work, but panelists agreed that the industry would benefit from an opportunity to apply technologies used in response to COVID-19 for other indications. Lewis pointed out that CGT innovators rely on partnerships to complete development tasks in acceptable timeframes. Although safety and product quality are not compromised, some risks are taken when multiple tasks must be performed in parallel and within modified timeframes from discovery to clinic. Thompson noted the importance and difficulty of having enough supplies (e.g., enzymes, lipids, and syringes) to generate hundreds of millions of doses of a vaccine in a short amount of time. “It’s amazing to do so much at the same time. You are doing clinical trials, but also doing late-stage manufacturing activities — as if everything is going to work. Typically, we [Pfizer] don’t take these risks, but now we are doing everything at the same time.”
Supply-chain concerns also was the subject of another conference panel titled “Raw Material, Viral Vector, and Supply Chain Considerations,” led by Christopher Bravery (Consulting on Advanced Biologicals). Panelists included Scott Burger (Advanced Cell and Gene Therapy), Christine Niederlaender (formerly Medicines and Healthcare Products Regulatory Agency, AMBR Consulting Ltd), Max Sellman (Aldevron), and Tom Walls (Spark Therapeutics). They pointed out that even before the pandemic, biopharmaceutical manufacturers faced problems with changing lead times and delays, especially for single-use assemblies and bulk media materials. The industry also is experiencing shortages of certified reference materials and problems with handling variability of activity in those materials. Suppliers must either buy reference materials or prepare them in house when a reference product is unavailable (which, as one panelist pointed out, is the case for most items in a bill of materials). Typically, good manufacturing practice (GMP) facilities conduct biological activity assays on materials when received (if a standardized assay is available). Some materials, however, cannot be tested against compendial standards, so those materials are difficult to source. How to ensure activity and consistency of materials such as cytokines is “an open question for which we don’t have an answer,” said Bravery. “But it is something that we need.”
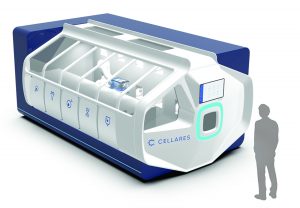
Cell Shuttle automated cell therapy
processing system from Cellares (https://www.cellares.com)
Innovation: Automated Closed Processing
CGT industry suppliers also have noticed the need for better process technologies. We spoke with Fabian Gerlinghaus, cofounder and chief executive officer at Cellares, about his company’s closed and automated, industrial-scale cell therapy manufacturing system.
Why is there a need for automated solutions in cell therapy processing? Manufacturing autologous cell therapies is laborious, failure-prone, and extremely difficult to scale. To produce a single autologous dose today, a team of trained personnel spends up to two weeks in cleanrooms executing about 50 manual processing steps while clocking about 80 hours of touch time. The complexity of scaling that out to treat tens of thousands of patients per year per drug is tremendous.
Hundreds of clinical trials on cell therapies are ongoing, and the FDA projects 10–20 approvals per year starting in 2025. Currently, the cell therapy industry has no viable solution to meet commercial-scale patient demands. It is imperative that manufacturers overcome bottlenecks and adopt solutions that enable them to make their products at large scale.
The Cell Shuttle system is a fully automated, end-to-end manufacturing platform. End-to-end automation applies to the entire manufacturing process from loading a patient’s cells to unloading cells that are ready for release testing or freezing before infusion into that patient. All steps in between are automated, including cell enrichment, cell selection, activation, gene transfer, expansion, formulation, and fill–finish. The system produces up to 10 patient doses simultaneously. By automating that process from beginning to end and increasing throughput by a factor of 10, we are confident that we can help our customers scale out to meet the needs of tens of thousands of patients per year per drug.
Does the system need to be placed in a specific environment? It is a completely closed system, readily deployed into an ISO 8 environment rather than an ISO 7 cleanroom. ISO 8 cleanrooms are roughly five times cheaper to operate and maintain per square foot than an ISO 7. The system reduces the amount of required cleanroom space by about 80% per patient compared with manual manufacturing methods. By automating the entire manufacturing process end to end, it further reduces the headcount needed by up to 75% for many cell therapy workflows. Overall, we expect to be able to reduce the per-patient manufacturing cost by up to 70% for most processes.
What information can be obtained during the process? Currently, manufacturers are manually generating about 500 pages of documentation for every individual patient dose during each run. The Cell Shuttle system automates the manufacturing process and generates significant amounts of in-process quality control (QC) data, which enables our software solution to largely autogenerate electronic batch records. The system provides real-time, in-process QC data on cell count, cell viability, cell size, acidity, dissolved oxygen, temperature, and level of metabolites and nutrients. The software can help scientists mine and analyze vast amounts of in-process data using machine learning algorithms to help improve their cell therapy manufacturing processes moving forward.
What is your company’s Early Access Partnership Program? The Early Access Partnership Program was developed to ensure that our manufacturing platform meets the needs of the cell therapy industry. Through this program, we are fostering partnerships with leading academic centers and commercial organizations to ensure the Cell Shuttle system’s market fit and broad applicability. Rather than developing this technology in the secrecy of our own laboratories, we’re taking a collaborative approach. We conducted our market research early, and at the same time we brought in several key partners, including the Fred Hutchinson Cancer Research Center and PACT Pharma. Those partners are providing us with crucial insight into their manufacturing workflows and providing feedback on the Cell Shuttle system’s hardware and software. Getting that feedback early in the design process when it is easier for us to incorporate into our agile hardware and software development processes is essential.
Innovation: Electronic Batch Management
As CGT manufacturers work through scalability concerns and successfully market new products, they will need to handle increasing amounts of data. We spoke with Kwok Pang, chief operating officer at Autolomous, about the CGT industry’s need for digitalization and better data management.
Why are digital solutions needed for cell therapy manufacturing? I’ve been in advanced therapies for the past 18 years, and I’ve seen the CGT industry’s growing need for scaling to large commercial scales and the need for better tools for managing data generated from those processes. Autologous therapy manufacturing, for example, in which an entire batch is made for one patient, generates a lot of critical manufacturing data. That data are generated for every patient.
Two years ago, Autolomous was founded to tackle data management challenges related to scaling up CGT processes. We commercialized an electronic batch manufacturing solution that helps to link process-generated data for all types of complex manufacturing workflows. Our AutoloMATE systems is supporting a dendritic-cell vaccine workflow, a chimeric antigen receptor (CAR) T-cell workflow, an allogeneic natural-killer (NK) cell workflow, and an induced pluripotent stem cell (IPSC) workflow with a US contract development and manufacturing organization. In all environments, data must be managed to reassure regulators that manufacturing scale up happens in a controlled manner with full auditability. That’s where our digital solutions come into play.
At what level do your software solutions apply? Can they be used with a company’s existing systems? We are engaging with CGT manufacturers that are still trying to optimize their processes and might be considering new unit operations in their workflows. We are offering a solution that provides control but also the flexibility to make changes. Development tools in our platform enable CGT manufacturers to adjust and make complete workflows themselves, without needing computer coding experience.
We’ve spent the past 18 months building seven integrations to suppliers across the ecosystem. Such integrations have been with unit electroporation devices and with flow cytometry analyzers. We also will be working with supply chain track-and-trace providers to integrate a hospital’s chain of custody and chain of identity for patient blood units along the manufacturing journey to manufacturing facilities and back to hospitals with finished drug products for infusion into patients.
How can such solutions help manufacturers understand their data? Many CGT manufacturers are quite early in the understanding of their data and in their identification of key quality attributes. In some cases, paper batch records still are being used. A typical paper batch record for a typical CAR T-cell workflow is about 100 pages long. An allogeneic product that runs for four weeks can generate about 400 pages.
Batch-to-batch trending or collation of results with the data are almost impossible with paper records. Our solution enables all data to be digital, which enables trending and tracking different attributes to help manufacturers identify critical quality attributes (CQAs). At the moment, CQAs are defined before clinical trials or manufacturing are conducted based on the best knowledge of a process. However, only selected attributes can be monitored using manual systems. In a digital process, data for attributes can be captured in a batch manufacturing record for trending.
We also are working on a new AutoMATE Assist module for batch verification downstream from manufacturing. It will prevent manufacturing bottlenecks from being pushed into batch verification tasks, leading to a backlog of products in freezers waiting for review. We have heard from CGT manufacturers that the product review process occupies a significant portion of their overall manufacturing timeframe. Often, that is because data must be collated before a review can happen. By integrating that process with other systems, data can be collected as it is generated. That provides a complete timeline showing when all information was generated, expediting and simplifying the collation of data and the review.
Data also must have end-to-end traceability. We leverage distributed-ledger technology (DLT or Blockchain technology) in our solutions. We connect each inbound piece of data to the previous piece of data. Our platform can show digitally that every piece of information captured has not been manipulated and was captured at a certain time point, thus enabling complete transparency of data capture.
Next Steps
Authors in this report discuss in detail other obstacles faced during cell therapy manufacturing and discuss the support available as the industry continues to mature.
Corresponding author Maribel Rios (maribel.rios@informa.com)
is managing editor, and Brian Gazaille is associate editor at BioProcess International, part of Informa Connect.
