Structural integrity of protein-based therapeutics is one of the major challenges in the biopharmaceutical industry. Multiple factors such as product stability, efficacy, and shelf life could be affected following minor changes in manufacturing process. Multiple biophysical methods employing spectroscopic and calorimetric tools can be used to analyse Higher Order Structure (HOS). Moreover, with an increasing demand for generating as much structural information as possible for regulatory submissions, a requirement for these analyses in a GMP environment is also important. This…
Analytical
Smart Sensors and Data Management Solutions for Modern Facilities
Bioprocess manufacturers continue to seek technologies for increasing productivity and shortening timelines from discovery to commercialization. Innovations such as high-throughput systems, automated platforms, and the latest clarification systems all have made processes efficient and robust. And with the increasing adoption of quality by design (QbD) principles, including the use of process analytical technologies (PAT), biomanufacturers are mitigating the risks of errors in their operations better than ever before. A critical part of mitigating risk is gathering meaningful process data and…
CMC Development Platforms and Outsourcing to Reduce Timelines
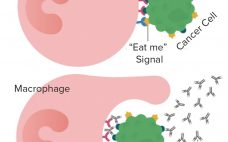
Forty Seven is a company developing novel therapies based on anti-CD47 and other immuno-oncologies. CD47 is called the “don’t eat me” signal that cancer cells give out to escape elimination by the body’s immune system. Qinghai Zhao, vice president of technical development and manufacturing, is one of the scientists working on the company’s magrolimab (5F9) monoclonal antibody that is designed to block the binding of the CD47 signal to the cell receptor SIRP-α while boosting the “eat me” signal that…
Introduction: Reporting from the Frontiers of Cell Line Engineering at BPI Europe and BPI West
Every biomanufacturing process begins with transfection of recombinant genes into pools of cells — followed by a succession of screenings from which will emerge (ideally) a single progenitor cell of the new production cell line. Cast aside will be those cells that do not uptake the correct genetic material, those incapable of thriving in bioprocess conditions, those that fail to produce recombinant protein at relevant levels, and those without demonstrated clonality and relative genetic stability. Over the past several years,…
Time Is of the Essence: Optimizing Cell Line Development
Cell line development is a critical upstream step for the manufacture of monoclonal antibodies (MAbs). Cells must be engineered to produce a biologic of interest, and the right clone must be selected to deliver material for preclinical and clinical studies. A high-producing cell line is then needed to support clinical studies and, ultimately, commercialization of the therapeutic. Please fill out the form below to read more. Corresponding authors are Aurore Poles, Global Technical and Compliance Expert, and Guillaume Plane, Global…
Biosimilarity Assessments: The Totality of Evidence Framework
Biosimilars are evaluated through comparisons with their reference products using abbreviated pathways that have evolved significantly over the past few years. Scientists and regulators now accept that some quality attributes can vary from batch to batch over a product’s lifecycle, even for reference products. Moreover, reference and similar biotechnology products can show differences in noncritical quality attributes but still demonstrate comparable efficacy and safety (1). Here we describe a similarity assessment approach that is also applicable to comparability of lifecycle…
Embedded Particles in Single-Use Bags: Risk to Bag Integrity and Drug Product Purity, or Only a Cosmetic Defect?
When using single-use systems (SUS) to process biopharmaceuticals, preventing drug product contamination from extractables and leachables (E&Ls) and embedded particulate matter (gel particles) in the polymer films used to make bioprocess bags is critical. Using a pressure burst test to assess film integrity, Sartorius Stedim Biotech’s Klaus Wormuth and colleagues compared Flexboy and Flexsafe samples with gel-particle-free materials to assess their potential for contamination. The results showed that only large (2–4 mm2) gel particles affected the burst test results, concluding…
A Faster Solution to Hybridoma Screening
Monoclonal antibodies are a rapidly growing class of therapeutics and are used in multiple clinical indications. This highly competitive landscape necessitates the need for rapidly developing next generation antibodies against new challenging targets with improved mechanism of action and pharmacokinetics. Current antibody screening tools such as ELISA, only report on binding to one antigen target at a time; hence these assays are time consuming and require large amounts of target protein. This case study highlights how the Intellicyt® iQue3 system…
Taking Your Molecule Through Process Validation
The dynamics of the biopharmaceutical industry to get innovative products to the client has evolved over the years. Studies have shown that by 2021, biologics and biosimilar products are projected to have higher growth than other pharmaceutical products. Following the industry trend, Avid Bioservices as a Contract Development Manufacturing Organization (CDMO) has helped numerous clients complete their process validations campaigns. Between 2016 to 2019, Avid has successfully completed six. This custom report will share some key factors to consider for…
Biopharmaceutical Characterization, Part 2: Applications and Strategies for Diverse Products — A Conference Report
Last fall, KNect365 brought together more than 250 analytical specialists to discuss biological assays and characterization of well-characterized biologics in Rockville, MD. Speakers from the US Food and Drug Administration joined experts from leading biopharmaceutical companies, service providers, and consultancies for case studies, regulatory interactions, sharing perspectives, and learning about emerging technologies. Part 1 of this report in January 2019 focused on the bioassay section of the meeting. Here in Part 2 sponsored by Sartorius, BPI’s senior technical editor reports…