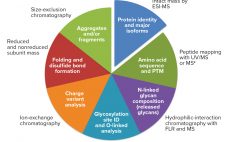
Biosimilars are evaluated through comparisons with their reference products using abbreviated pathways that have evolved significantly over the past few years. Scientists and regulators now accept that some quality attributes can vary from batch to batch over a product’s lifecycle, even for reference products. Moreover, reference and similar biotechnology products can show differences in noncritical quality attributes but still demonstrate comparable efficacy and safety (1). Here we describe a similarity assessment approach that is also applicable to comparability of lifecycle…
Author Archives: Jose Menezes
Risk Management of Biopharmaceutical Operations: End-to-End and Over Lifecycle
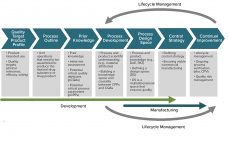
Biopharmaceutical manufacturing processes that were developed before the implementation of quality by design (QbD) typically use control strategies that are not founded on a formal understanding of criticality. Thus, manufacturers of “legacy” products lack the understanding of critical process parameters (CPPs) and critical quality attributes (CQAs). Introducing such elements to a legacy biologic drug product filing aligns fully with expectations described in the ICH Q12 guideline (e.g., increased process understanding and better risk mitigation strategies) (1). Here we discuss how…
Is the QbD Toolbox Ready for Cell and Gene Therapies? Integrating Patient Outcomes into Manufacturing Cell and Gene Therapy Bioproducts
In their lifecycle development and manufacturing models, biotechnology products and the biopharmaceutical industry have been founded on principles originating from the pharmaceutical small-molecule industry. Such principles define clinical programs that establish risk benefits of a dosage and its delivery system on healthy individuals and patients. A company then develops a process to manufacture that product consistently over several years. Product quality attributes set through manufacturing controls are expected to ensure patient outcomes in terms of safety and efficacy and deliver…