Recent approvals of oligonucleotide therapeutics are a clear signal for optimism for this product class. This is supported by the strength of the current pipeline which has over 180 active oligonucleotide clinical programs in various phases of development. Improvements in analytical technology and know-how have played a key role in enabling suitable characterization and quality control strategies to overcome the difficulties associated with testing these complex molecules. Despite the lack of dedicated regulatory guidelines related to characterization or quality control,…
Analytical
Analytical Tools to Improve Decision-Making During Product Development
Speed to clinic testing — and then speed to market — are highly significant metrics for companies developing biopharmaceuticals. By increasing the pace of drug development, these companies can reduce costs, obtain revenues early, and establish commanding positions in the market relative to their competitors. High-throughput development tools have contributed much to the acceleration of drug development in recent years. Such technologies enable the testing of many process parameters in parallel. Combining them with multifactorial “design of experiment” (DoE) analysis…
Visible Particulate Matter in Single-Use Bags: From Measurement to Prevention
Parenteral pharmaceuticals must be “essentially free” from visible particulate matter (1). In the production of biopharmaceuticals with single-use systems (SUS), biocompatibility requires controlling interactions between drug substances/products and SUS surfaces to ensure drug product quality and patient safety with regard to extractables/leachables and particulate matter. Any particulate matter stuck to fluid-contacting surfaces of process components could wash off and contaminate process fluids. Depending on system configuration, a final drug product could be at risk for particulate matter from SUS. Risk…
Emerging Tools for Exosome Purification and In-Process Monitoring
This eBook introduces new analytical approaches that enable in-line chromatographic detection of exosomes. One approach can discriminate extracellular vesicles from nonvesicle contaminants, and one potentially can discriminate exosomes from other vesicles. Examples illustrate how they enable development of more effective and better documented purification methods. The special qualifications of monolithic chromatography media for exosome purification are discussed. New process tools designed to accommodate some of the special challenges of exosome purification are introduced. Exosomes represent one of several species of…
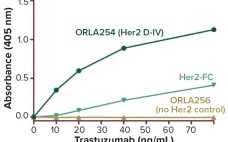
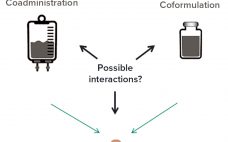
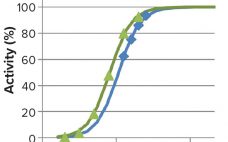
Analytical Strategies for Fixed-Dose Coformulated Protein Therapeutics
Coformulation of two or more proteins in a single formulation is an emerging approach to delivering multiple biotherapeutics that previously have been administered in sequence. This approach brings multiple benefits to all stakeholders. Foremost for patients, the primary benefits are combined therapeutic effects and improved convenience (e.g., fewer administration events). Healthcare providers see logistical benefits and decreased risk of medical errors. Additionally, coformulations also simplify manufacturing logistics, reduce costs of packaging and distribution, and provide new opportunities for product portfolio…
Recommended Practices for Assuring Integrity of Single-Use Systems
The increasing uptake of single-use technologies (SUTs) in critical current good manufacturing practice (CGMP) processes and applications has made their integrity a critical quality attribute (CQA) for both suppliers and end users of such systems. Current regulations focus on final packaging, however, without taking into account the unique aspects of assemblies used in bioproduction. Ongoing initiatives include revision of PDA TR 27 (1) and creation of A STM workstreams (2, 3) to propose good practices for the integrity of single-use…
Points to Consider in Quality Control Method Validation and Transfer
The concept of an analytical lifecycle has been well received in the biopharmaceutical industry. In 2016, the US Pharmacopeia (USP) advocated for lifecycle management of analytical procedures (1) and defined its three stages: method design development and understanding, qualification of the method procedure, and procedure performance verification. The US Food and Drug Administration (FDA) has published guidance on process validation with a similar division into three stages: process design, process performance qualification, and process performance verification (2). For a manufacturing…
Apparent Matrix Effects in an Iduronate 2-Sulfatase Specific Activity Assay
The recombinant fusion protein SHP631 consists of a chimeric monoclonal antibody binding to human insulin receptor and iduronate-2-sulfatase (I2S). This product is being developed as an enzyme replacement therapy to treat cognitive symptoms of Hunter’s syndrome. Because the current therapy (idursulfase, brand name Elaprase from Shire) cannot cross the blood–brain barrier (BBB), SHP631 is being developed to do so, enabling the presence of I2S in the brain. The enzymatic activity of this molecule is measured using the substrate 4-methyl umbelliferyl-α-L-idopyranosiduronic…
Qualitative and Quantitative Host Cell Protein Analysis Using Mass Spectrometry
Host cell proteins (HCPs) originate from host organisms that are used to produce biopharmaceutical products. They are in-process contaminants that must be minimized during downstream process operations. According to regulatory agencies, the maximum permitted level of total HCP in a biopharmaceutical product is 100 ng per mg (100 ppm) (1). HCPs can decrease drug efficacy and pose a risk to patient safety because they can bring on undesirable immune responses. Thus, HCPs are a critical quality attribute that should be…
Using Slope Spectroscopy Methods: Risk Assessment and Cost Savings
The biopharmaceutical industry’s need for rapid, accurate concentration measurements of protein-containing products is critical. The protein-concentration assay measures ultraviolet absorption at 280 nm (A280) and usually is performed both as an in-process test and for product-release testing. The SoloVPE system can analyze samples across a wide range of target concentrations without the need for labor-intensive and error-prone dilutions. Slope Spectroscopy methods provide companies with a universal platform for determining protein concentration for all in-process, clinical, and commercial methods. During in-process UV…