The process analytical technology (PAT) and quality by design (QbD) guidelines promoted by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) support the idea that quality cannot be “tested into” a biologic product but must instead be part of its process design. Seamless integration of analytical data with bioprocess monitoring and control is crucial to understanding a process and overcoming manufacturing challenges that arise in the course of development. Monitoring of product quality attributes (PQAs)…
Analytical
Multivariate Data-Driven Modeling for Continued Process Verification
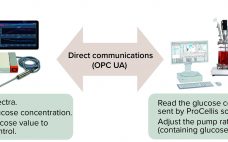
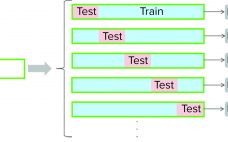
Continued process verification (CPV) is an integral part of process validation for the manufacture of human and animal drugs and biological products (1). It is designed to meet three primary goals: maintain a validated state of products, their processes, and related systems; enable continuous process improvements; and meet regulatory requirements for life-cycle validation. A CPV program for a biologic product entails regular collection of data related to critical process parameters (CPPs) and critical quality attributes (CQAs) and the preprocessing, analysis,…
Analysis of Trace-Level, High-Risk HCPs: Proteomics Advances for Preventing Degradation of Polysorbates in Biotherapeutic Formulations
Polysorbate-80 (PS-80) and polysorbate-20 (PS-20) are used widely in formulation of biotherapeutic products for preventing surface adsorption and as stabilizers against protein aggregation (1). Degradation of polysorbates can cause turbidity and potential formation of subvisible particles mainly consisting of poorly soluble hydrophobic free fatty acids (1). Polysorbate degradation is an industry-wide challenge both in biotherapeutics processing and formulation development. The risk of such degradation increases with higher cell densities and greater expression titers in bioprocessing, as well as with higher…
Tools to Support COVID-19 Patient Testing
To prepare adequate healthcare measures in the case of a pandemic, vast numbers of people must be tested in order to understand the dynamics and behavior of the infection cycle. The medical staff working under extreme circumstances need basic but reliable laboratory supplies to help contain the pandemic. Read this special report to discover two solutions for COVID-19 patient testing that comply with guidelines from the US Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO). The…
Accelerating the Development and Manufacture of Therapeutics Using the Octet Platform
The high costs of therapeutic discovery, development, and manufacture require improved process efficiencies and economics. Analytical tools that eliminate the need for reagent labeling and enable real-time data visualization save development time and improve efficiencies during process development. The Octet biolayer interferometry (BLI) platform and assays can be used throughout process development and manufacturing, including cell-line development, clone selection, and dynamic binding capacity (DBC) determination for affinity purification columns. The ability of the Octet BLI platform to monitor binding interactions…
eBook: Product Variants in Bioprocesses
Product variants are contaminants because they bear properties that are different from those of desired biological products with respect to activity, efficacy, and safety. Thus, such variants can compromise product quality and consistency. In this eBook, Yuval Shimoni explores different types of variants — including primary-sequence variants, undesirable posttranslational modifications, aggregates, and degraded proteins — and explains how their presence can diminish the performance and quality of drug substances and products. He also discusses how product variants form at different…
eBook: Process-Related Impurities — Emerging Strategies for Detection, Identification, and Management of Host-Cell Proteins
Host-cell proteins (HCPs) represent a major class of process-related impurities (PRIs) that are generated during biopharmaceutical manufacture. Although the vast majority of such proteins are removed from a drug substance during downstream purification, residual HCPs can remain in a finished drug product. Even in minimal concentrations, copurifying HCPs can pose safety risks and compromise protein-product yield, efficacy, and stability. Thus, regulatory agencies consider the presence of HCPs to be a critical quality attribute (CQA). Sufficient clearance of these impurities helps…
Contractor Perspectives: Best Practices for Transfer, Handling, Testing, and Storage of Cell Banks
For comments about how contract development and manufacturing organizations (CDMOs) manage their cell-banking quality assurance (QA) practices. I contacted long-time member of BPI’s Editorial Advisory Board Scott M. Wheelwright, PhD, for his perspectives. Wheelwright brings many years of experience to this discussion, with insights into the evolution of technologies and practices extending back to the early launch of the biopharmaceutical industry. Currently, he provides consulting support for companies with manufacturing and sourcing in China and other Asian countries. He also…
Measuring Viral Titer in AAV-Mediated Gene Therapy Using a PCR Technique for Absolute Quantitation
Gene therapies have reemerged as promising treatments to a number of genetic illnesses. Nearly 400 gene therapy clinical trials are recruiting or ongoing in the United States for diseases such as hemophilia and spinal muscular atrophy (1). The primary vehicles used today to deliver such therapies to patients’ cells are viral vectors such as adenoassociated viruses (AAVs), but producing biologically active vectors for gene therapy can be problematic. One difficulty is generating vectors at the correct concentration to yield a…
Analytical Methods for Cell Therapies: Method Development and Validation Challenges
Advanced-therapy medicinal product (ATMP) characterization and analysis play important roles in providing chemistry, manufacturing, and controls (CMC) information for regulatory applications as well as in supporting product-release and stability studies. Each type of advanced therapy presents different analytical development challenges, so each requires specific characterization, potency, purity and identity assays. Variability in cells and among patients, multiple and complex mechanisms of action (MoAs), a general lack of readily available reference materials, and complicated analytical methods and instruments underlie the major…