In many ways, patient-specific cell therapies (PSCTs) are still the “new kid on the block” in medicine. Researchers, therapeutic developers, manufacturers, regulators, and payers are still exploring and developing an understanding of the powerful benefits and unique challenges associated with this growing industry. As we all become more familiar with PSCTs, an evolution will need to occur — as it has for automobiles, computers, and every technological advance in human history — for these therapies to become widely adopted, cost-efficient,…
Search Results for: regenerative medicine
Emerging Platform Bioprocesses for Viral Vectors and Gene Therapies
Recent advances in molecular biology are expediting genomic sequencing to underpin precision medicine. Such progress is positioning gene and gene-modified cell therapy on the cusp of an extraordinary revolution in patient care for presently unmet medical needs — and a new therapeutic class that could rival monoclonal antibodies (MAbs) in importance. However, despite substantial strides made in clinical trials, the bioprocessing community is struggling to fulfill growing demands for biomanufacturing capacity to make gene and gene-modified cell therapies — including…
Automation of CAR-T Cell Adoptive Immunotherapy Bioprocessing: Technology Opportunities to Debottleneck Manufacturing
Continued clinical efficacy demonstrations of cell-based immunotherapies (iTx) such as chimeric antigen receptor T cell (CAR-T) therapies has made the prospect increasingly likely of an immunotherapy product achieving conditional market authorization in the short term. For example, Novartis and the University of Pennsylvania’s lead candidate (CTL019) for treating a range of hematological malignancies received breakthrough status from the US Food and Drug Administration (FDA) in 2014, permitting access to an expedited drug development pathway for high unmet medical needs (1).…
Development of a Novel Cell-Separation Platform: Discussion with Quad Technologies CEO Sean Kevlahan
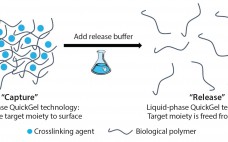
Releasing and separating cells from surfaces and capture molecules are critical steps in cell therapy development. Research into such therapies as chimeric antigen receptor T cells (CAR-T) cancer therapies and stem-cell regenerative medicines demand the isolation and purification of viable and functional target cells. A number of cell-separation strategies can be used to produce such cells, but they are not able to deliver the required efficiency or scalability and can also cause damage to cells or affect their phenotype. Since…
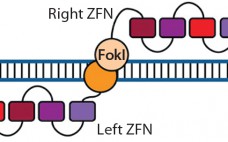
Therapeutic Gene Editing: Tools to Facilitate Basic Science or Stimuli for a Paradigm Shift in Biomanufacturing?
Historically, fundamental science and process engineering were separated by distinct vernaculars and a decade or more in the translation pathway of candidate therapeutics from laboratory to bedside (1). This crude metric holds true for the origins of the modern pharmaceutical industry, namely fine chemicals that supported the high-margin small molecules that constitute the majority of the pharmacopoeia even today. But as illustrated by deeply interwoven careers, companies, and technologies — including those related to monoclonal antibodies (MAbs) — that classic…
From the Editor
Included with this issue is our first of two supplements this year focused on cell therapies. It has been fascinating to watch this journey toward maturation of advanced therapeutics. Some early assumptions that commonalities with protein production would resolve manufacturing problems are being tempered now that we know more about the unique demands of cell and gene therapy development. Still, the future looks brighter for these new modalities. As Invetech’s Richard Grant (global VP, cell therapy) noted in his plenary…
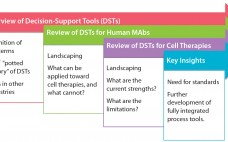
Decision-Support Tools for Monoclonal Antibody and Cell Therapy Bioprocessing: Current Landscape and Development Opportunities
Industrial-scale manufacturers in a number of fields — from automobiles to biotherapeutics — have long relied on powerful computational and mathematical tools to aid in the scale-up, optimization, quality control, and monitoring of product development (1–5). Typical process pathways are highly multifactorial, with numerous branch points, feedback steps, instrumental attributes, and target parameters. Moreover, margins for error are minimal for most industrial processes, requiring high standards of precision from industrial and operational pathways (6). For those reasons, the complexity of…
From the Editor
Along with products and processing operations moving upstream and downstream, they also move toward the mainstream. The ramifications can become so interconnected that it is hard to discern cause and effect. One example from the past 10 years is single-use materials moving into larger scale processing. That in turn has driven much exploration into flexible operations and variations of continuous processing — neither particularly new concepts, but both now taken into more (potentially) economical directions. Related discussions are bringing heightened…
Stem Cell Summit 2016
The Stem Cell Summit 2016, will be held on April 25-27, 2016 in Boston, MA. GTCbio’s Stem Cell Summit provides cutting-edge information on developments in all areas of stem cell research from bench to bedside, including the biology, medicine, applications, regulations, product development, and the commercialization of stem cells. Recent developments in pre-clinical and clinical trials of stem cell therapy, regenerative medicine and tissue engineering, cancer stem cells, immunotherapy, stem cell reprogramming, regulatory policies regarding stem cell research, and manufacturing…
Quantitative Risk Assessment of Bioaccumulation Attributable to Extractables and Leachables in Cellular Immunotherapy Biomanufacturing
Precious patient samples, contamination concerns, and limited product purification options have compelled manufacturers of cellular immunotherapies (iTx) such as chimeric antigen receptor T cells (CAR-T) and T-cell receptor (TCR) technologies toward the disposables industry. Such companies are implementing single-use technologies (SUTs) almost exclusively (1). But despite the dominance of disposable bioprocess platforms and their extraordinary growth in the iTx marketplace, researchers have made limited efforts to understand the perennial and critical bioprocessing risks of leachables and extractables. Here we outline…