Single-use fermentors (SUFs) offer dramatic advantages over traditional stainless-steel clean-in-place (CIP)/sterilization-in-place (SIP) fermentors. Single-use technology eliminates the need for steam, chemicals, and water to clean and sterilize stainless vessels, which provides a direct reduction in capital cost and environmental-impact mitigation. Single-use platforms also increase equipment and process flexibility significantly, making it possible to switch campaigns from one product to another in minimal time, while eliminating cleaning and associated validation steps (1). Both Cytiva and Thermo Fisher Scientific offer SUFs that…
Upstream Processing
Posttranslational Modifications and Their Control in CHO Culture
The Chinese hamster ovary (CHO) cell line was first established by Theodore Puck in the 1950s and was used mainly for cytogenetics studies (1). Since the 1990s, CHO cells have increased in popularity as expression host cells because they can be adapted easily into suspension culture and genetically modified. The CHO cell line also has a human-like glycosylation profile (2–4). Therapeutic proteins undergo different posttranslational modifications (PTMs) during manufacturing. Some modifications can lead to undesired effects such as decreased stability,…
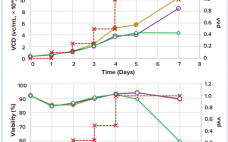
High-Yield Production of rAd26-S for Sputnik V Vaccine Component I: An Optimized Process in a Scalable Shaken Bioreactor
The recent outbreak of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) led to the development of different vaccine approaches worldwide to prevent the coronavirus disease 2019. The first registered vaccine on the market was the Sputnik V product based on two recombinant adenoviral vectors (Ad5 and Ad26). The product has received approval in 70 countries by several national and regional regulatory authorities, meanwhile. Though the availability of SARS-CoV-2 vaccines in most developed countries is not an issue any longer, other…
Tris, a Critical Raw Material: Improving the Quality and Consistency of Supply
ANGUS Life Sciences is the world’s largest supplier of tromethamine buffers and the only manufacturer of the tris molecule based in the Western hemisphere. The company sells directly to biopharmaceutical customers and contract manufacturing organizations as well as to reprocessors who repackage the chemical or process it into different grades and derivatives. After recent expansions in both the United States and Germany, the company now boasts dual-source manufacturing capabilities for its highest-purity tris products and is confident about its ability…
Optimizing the AAV Transfection Process in Suspension Cells
Given the potentially curative nature of gene and gene-modified cell therapies and the successful launches of several such products over the past five years, markets and investments are growing significantly in the sector. In the United States alone, the US Food and Drug Administration has granted approval for several genetic therapies, including Strimvelis (autologous CD34+, developed by GlaxoSmithKline and later sold to Orchard Therapeutics) in 2016 Luxturna (voretigene neparvovec, Spark Therapeutics), Yescarta (axicabtagene ciloleucel, Kite Pharma/Gilead), and Kymriah (tisagenlecleucel, Novartis)…
Viral Safety of Viral Vectors:
Special Concerns Arise When the Virus Is the Product
As anyone who has focused on host-cell proteins as process contaminants can tell you, trying to purify a specific type of molecule from a large mixture of many similar molecules is like trying to find a few particular needles in a huge pile of varied needles. The same could be said for purifying viral vectors from cell culture fluids. When viruses are the products, unwanted viruses are contaminants that must be separated away — or better yet, prevented from being…
A Strategic Approach to Selecting the Optimal Process Intensification Scenario
Current demands placed on the biopharmaceutical industry are pushing manufacturers toward process intensification, an approach that modifies unit operations or an entire manufacturing process to optimize efficiency. Three common intensification scenarios in upstream processing are seed-train intensification (usually at the n – 1 stage), concentrated fed-batch production, and dynamic perfusion (at the production bioreactor stage). In downstream processes, intensification strategies typically involve moving from single- to multicolumn chromatography. Biomanufacturers can realize several kinds of improvements from intensified processing, including reductions…
Transfection Best Practices for AAV Gene Therapy Programs
As viral vectors continue to push gene therapy innovations closer to market, many researchers are setting their sights on optimizing transfection, the process of delivering corrective genetic material into cells. It’s not just a question of how to transfect them, but also how to do so efficiently and at high volumes. Approaches that work for one cell line might not perform well for others, and transfection protocols can have different implications for scalability and cost during production for clinical trials.…
Recombinant Proteins for Cell and Gene Therapy Research: A Conversation with Shenandoah Biotechnology
Recombinant proteins such as growth factors and cytokines are essential for cell therapy, gene therapy and regenerative medicine research, development and manufacturing. These proteins are critical in the production of desired cell types and subsequent differentiation of cells, to deliver the desired effect. Founded 15 years ago, Shenandoah Biotechnology applies a proprietary method of folding and purifying recombinant proteins from both bacterial and mammalian systems to enable cost-effective, large-scale production of Cell Therapy Grade proteins to support these groundbreaking treatments.…
Spontaneous Infection: Did You Leave the Back Door Open to Your Cultivation Suite?
Manufacture of biopharmaceuticals using mammalian cells inherently incurs a risk of viral contamination during cell cultivation. If introduced, viruses can infect and replicate in cells used to produce a therapeutic protein or vaccine. The consequences of such contaminations can be dramatic. Not only can a company lose contaminated batches, but it also faces potentially extensive root-cause investigations, facility cleanup efforts, and introduction of preventive measures. Until contamination issues are resolved adequately, production should not be resumed, and facility downtime brings…