Biosimilars are biologically derived pharmaceuticals intended to have clinical similarity to a legally marketed innovator product when that product’s patent or market exclusivity has expired. By contrast with generic small-molecule drugs, clinical performance of a biologic pharmaceutical is a function of its structural complexity and higher-order structure (HOS). Biomanufacturing controls of such complex products cannot fully ensure chemical similarity between an innovator product and putative biosimilar because minor differences in chemical modifications and HOS can significantly alter a product’s safety…
MAb
Addressing the Challenges of Developing Biopharmaceutical Drugs
The biopharmaceutical industry is enjoying considerable success. Its products account for about a fifth of world pharmaceutical revenues, which are growing at twice the pace of those generated by most traditional chemically synthesized drugs. Biopharmaceuticals populate the list of best-selling drugs, and a number have achieved blockbuster status. Biotechnology stocks have outperformed the general market as investment has flowed into the industry. As with other highly profitable markets, the market for biopharmaceuticals has become increasingly competitive. Reflecting this fact, in…
Standardized Economic Cost Modeling for Next-Generation MAb Production
Historically, in generating material for clinical testing during antibody process development, emphasis was placed on efficacy, product quality, regulatory compliance, and speed. As the biopharmaceutical industry has matured (and with increasing competition), emphasis has shifted toward cost optimization and manufacturability. Reducing the costs of medicines for patients and payers (thereby broadening access to drugs) is now a key driver during development of new therapies as well as modernizing processes for existing molecules. Cost reduction includes providing robust manufacturing processes that…
Quality By Design for Monoclonal Antibodies, Part 2: Process Design Space and Control Strategies
Process design space and control strategy are two fundamental elements of quality by design (QbD) that must be established as part of biopharmaceutical development and regulatory filings. Like all of QbD, they are interconnected and iterative. Both are based on knowledge gained during product and process development — but both need to be in place (in a potentially very limited form) when a company begins to manufacture drug substance for clinical trials. Part 1 of this discussion appears on pages…
Performance Qualification of a Single-Use, Stirred-Tank Bioreactor with CHO-S Cell Culture
The increasing role and importance of cell culture in biophamaceutical manufacturing has led to considerable research and development (R&D) into bioreactor design and performance in recent years. As a result, a greater understanding of bioreactor fluid dynamics and critical physical parameters is now essential to maximize cell growth and productivity. Stirred-tank bioreactors are especially important in this development process because of their favorable properties in areas such as mixing efficiency and homogeneity, energy transfer, and scalability. The design and manufacture…
Optimizing Continuous Monoclonal Antibody Polishing By Using Coupled Unit Operations
The biopharmaceutical industry is under a great deal of pressure to modernize manufacturing to meet the challenges of production at vastly different scales for niche drugs as well as for expected massive blockbusters, biosimilars, and regional manufacturing. To address these challenges, the biopharmaceutical industry is embracing process intensification through single-use and continuous processing technologies. Implementing these technologies offers increased productivity and manufacturing flexibility and reduces the footprint, capital outlay, and operating costs. Pall Life Sciences has developed several technologies designed…
Critical Factors for Fill–Finish Manufacturing of Biologics
Over recent decades, protein-based therapeutics have emerged as key drivers of growth in the pharmaceutical industry. Drug development pipelines have filled with biologics, and a handful of monoclonal antibody (MAb) products have become some of the best-selling drugs around the world. Production of biotherapeutics is often challenging because of the inherent instability of these large, complex molecules. Their fragile nature has forced manufacturers to change how bulk drug substances (BDSs) are handled and final drug product is formulated, sterile filtered,…
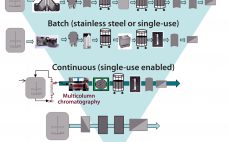
Special Report on Continuous Bioprocessing: Upstream, Downstream, Ready for Prime Time?
Once an engineering curiosity and smallscale laboratory technique, continuous bioprocessing has evolved in just a few short years to a topic of intense and increasing interest to most bioprocessors. Critics point to a steep learning/adoption curve, but that is nothing new in biomanufacturing.Andrew Zydney is a distinguished professor of chemical engineering at Pennsylvania State University. He has noted these challenges facing continuous processing: commercially unproven unit operations (especially downstream), a lack of equipment robustness, sterility concerns, and uncertain development timelines…
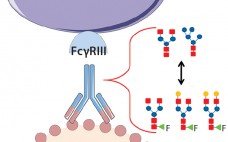
Fucosylation of a Therapeutic Antibody: Effects on Antibody-Dependent, Cell-Mediated Cytotoxicity (ADCC) Potency and Efficacy
Product quality attributes are critical for the functionality and manufacturability of therapeutic antibodies. They can be significantly influenced by a number of production process parameters, such as cell culture media. The composition of growth and feed media can influence antibody glycosylation, including the concentration of ammonia, glutamine, glucose, and metal ions (1, 2). Thus, it is critical during media development and optimization to monitor and consider a culture medium’s impact on glycosylation. For therapeutic antibodies whose mechanism of action includes…
Rapid Formulation Development for Monoclonal Antibodies
Monoclonal antibodies (MAbs) are at the focal point of biologics development. Many of the best-selling drugs are therapeutic MAbs or related proteins (1–2). The combined world-wide sales from MAbs will be nearly US$125 billion by 2020 (3). About 50 MAb products treating a range of diseases have been approved in the United States or Europe. With the large number of MAbs progressing through discovery, biomanufacturers need to accelerate process development and move projects rapidly into clinical manufacturing (4–5). Formulation development,…