Opportunities for establishing strong biopharmaceutical capabilities are expanding across the globe. This e-book seeks to encapsulate the current state of emerging markets/countries, tracing key elements above and offering examples to show where (in the world) the biopharmaceutical industry is expanding and securing its footholds. Generally, to succeed in these markets, foreign companies must exercise efficient resource management and control, show creativity and receptiveness to cultural differences, develop new strategies, and manage expectations. Working with local partners can provide access to…
Regulatory Affairs
Regulation, Analytical, and Process Issues with Leachables: Toward Harmonization for Latin America with Europe and North America
The pharmaceutical industry follows strict regulations regarding impurities, including process-related leachates. Plastic manufacturers use hydrophobic, nontoxic additives for manufacturing containers for use in the pharmaceutical and food industries. However, some issues about dealing with such impurities are not yet resolved. In developing countries, regulators are working on guidelines to help local companies ensure characterization of impurities. In this exclusive editorial eBook, authors from Mexico describe some issues related to plastic leachables in the context of ongoing efforts to harmonize regulations…
An Industry Proposal for Change Notification Practices for Single-Use Biomanufacturing Systems
Current practices for change notification in the biopharmaceutical industry are neither efficient nor conducive to accelerating the adoption of single-use systems (1, 2). Drug manufacturers (end users) often observe that supplier change data packages lack technical content or detail and that the time allowed for change implementation is too short. Occasionally, customers (end users or next tier in supply chain) learn of changes after the fact, possibly even by happenstance. Suppliers, however, can find the potential affect of a change…
Opportunities and Challenges in Biosimilar Development
A biosimilar biotherapeutic product is similar (but not identical) in terms of quality, safety, and efficacy to an already licensed reference product. Unlike generic small molecules, it is difficult to standardize such inherently complex products based on complicated manufacturing processes. Table 1 describes the main differences between biosimilar and generic drug molecules. The global biosimilar market is growing rapidly as patents on blockbuster biologic drugs expire (Table 2) and other healthcare sectors focus on reduction of costs. Biologics are among…
Implementing Quality By Design in Analytical Development: A Case Study on the Development of an Anion-Exchange HPLC Method
The concept of quality by design (QbD) initially was outlined in ICH Q8 guidance for drug-product development and later in Q11 for drug-substance development (1, 2). Since then, the QbD concept was further expanded to the development of analytical methods. FDA issued a 2015 guidance on analytical procedures and method validation for drugs and biologics (3). Although the agency did not explicitly state the requirement for implementation of QbD in analytical method development, the concept is embedded in its section…
Introduction: Tackling the Technical and Regulatory Challenges of Biosimilar Development
In a just a few years, the biopharmaceutical industry has gone from questioning the feasibility of “follow-on biologics” (around the time of BPI’s first issues) to fearing them (when we published our first supplement on the topic in 2013) to the acceptance and strategizing of today. Perhaps because of its more socialized medicine, Europe led the way in biosimilar regulation and approved its first such product nearly 10 years before the first US biosimilar launch in 2015. In between came…
The Clinical Side of Biosimilar Development
Biosimilars have become common on pharmacy shelves in Europe. The first biosimilar product — Sandoz’s Omnitrope version of Lilly’s Humatrope (somatropin) — was approved by the European Medicines Agency (EMA) in 2006. In the decade that followed, more than 20 biosimilars have gained regulatory approval in Europe. The first biosimilar monoclonal antibodies (MAbs) — comparators to Janssen’s Remicade (infliximab) — were approved in 2013. The pace of approvals in the United States has been much slower. The US Food and…
Evolving Bioassay Strategies for Therapeutic Antibodies: Essential Information for Proving Biosimilarity
The modern age of biologics began 35 years ago with the approval of Lilly’s Humulin product — a biosynthetic form of human insulin derived from recombinant DNA and microbial cell culture (1). Today, about a quarter (27%) of the drugs approved yearly by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) are biopharmaceuticals: primarily monoclonal antibodies (MAbs), but also vaccines, blood products, and (recently), advanced therapies based on genes and cells. A decade ago, the average…
CMC Strategy Forum on Combination Products for Biopharmaceuticals: Emerging Trends in Development, GMPs, and Regulatory Expectations
On 26 January 2015, CASSS hosted a program in its ongoing series of semiannual Chemistry, Manufacturing, and Controls (CMC) Strategy Forums at the Mayflower Hotel in Washington, DC. Since this series’s inception in 2002, each installment has focused on one of a wide array of topics spanning the fields of biopharmaceutical product development, manufacturing, analysis, quality, and regulation. For this forum, the program committee chose to devote a full program to a topic that was previously the focus of an…
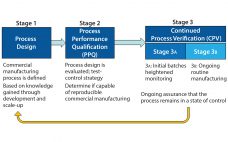
CMC Forum: Evolution of Biopharmaceutical Control Strategy Through Continued Process Verification
As defined in the ICH Q10 guideline, a control strategy is “a planned set of controls, derived from current product and process understanding, that assures process performance and product quality” (1). Every biopharmaceutical manufacturing process has an associated control strategy. FDA’s 2011 guidance for process validation (2) describes process validation activities in three stages (Figure 1). A primary goal of stage 1 is to establish a strategy for process control that ensures a commercial process consistently produces acceptable quality products.…