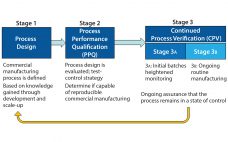
As defined in the ICH Q10 guideline, a control strategy is “a planned set of controls, derived from current product and process understanding, that assures process performance and product quality” (1). Every biopharmaceutical manufacturing process has an associated control strategy. FDA’s 2011 guidance for process validation (2) describes process validation activities in three stages (Figure 1). A primary goal of stage 1 is to establish a strategy for process control that ensures a commercial process consistently produces acceptable quality products.…
Author Archives: Stefanie Pluschkell
CMC Strategy Forum Special Focus Series: Part 2 Product-Related Impurities, An Overview
Introduction by Cheryl Scott The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of forum meetings are published in BioProcess International and on the CASSS website. This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated environment…
Multiproduct Facility Design and Control for Biologics: Challenges and Considerations
Multiproduct facilities are increasingly integral to corporate biologics network and supply chain strategies. Manufacturing capacity strategies ensuring appropriate facility design and procedural controls to manage the risks of producing multiple products are critical to the successful deployment of commercial and clinical supply plans. A Chemistry, Manufacturing, and Controls (CMC) Strategy forum was held in Bethesda, MD, in August 2011 to highlight various challenges, risks, and control strategies associated with multiproduct facilities. Multiproduct strategies for the manufacture of a variety of…
Expanded Change Protocols: Benefits, Cost Considerations, and Regulatory Views
The US FDA Office of Biotechnology Products’ quality by design (QbD) pilot program defines an expanded change protocol (eCP) as a particular type of comparability protocol that will “describe the quality by design, risk- based approach linking attributes and processes to product performance safety, and efficacy” (1). Sponsors have explored a wide range of potential applications for eCPs (e.g., movement within or beyond an established design space, site transfers, and additional process modifications supported by either a QbD or traditional…
Multiproduct Facility Design and Control for Biologics
Multiproduct facilities are increasingly integral to corporate biologics network and supply chain strategies. Manufacturing capacity strategies ensuring appropriate facility design and procedural controls to manage the risks of producing multiple products are critical to the successful deployment of commercial and clinical supply plans. A Chemistry, Manufacturing, and Controls (CMC) Strategy forum was held in Bethesda, MD, in August 2011 to highlight various challenges, risks, and control strategies associated with multiproduct facilities. Multiproduct strategies for the manufacture of a…