Significant growth of the cell and gene therapy (CGT) pipeline in recent years demonstrates the enormous potential of these modalities to treat or even cure otherwise intractable diseases. Several CGT products have been approved for clinical use over the past five years. More than 75 such products have come to the market around the world so far. They include chimeric antigen receptor (CAR) T-cell therapies that involve genetic engineering of patient cells ex vivo as well as in vivo gene therapies…
Business
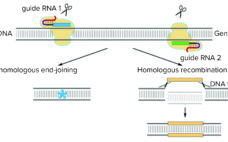
The CRISPR Saga (So Far)
Since January 2016 (with a brief interlude as described below), the Patent Trial and Appeal Board has been attempting to adjudicate the proper inventorship of CRISPR technology in (to date) six separate patent-interference proceedings. (Scientific “priority has been decided, for now, by the awarding of the Nobel Prize in Chemistry to Jennifer Doudna and Emmanuelle Charpentier in 2020; see the “Priority Claims” box). CRISPR-based gene editing was hailed as the “Breakthrough of the Year” in 2015 (1), and the scientific…
Orphan Drug Designation: Securing the Significant Benefits
When preparing an application for orphan drug designation (ODD) in the European Union (EU), you need to consider key eligibility criteria that are different from those required by the US Food and Drug Administration (FDA). One factor is justifying the “significant benefit” (defined below) of your drug product over existing therapies for an orphan condition. Significant benefit contributes considerably in securing a successful ODD application if the correct approach is taken — as described in two European Medicines Agency (EMA)…
Maximizing European Market Access: Guidance for Young Biopharmaceutical Companies
Achieving efficient and profitable market access for next-generation pharmaceutical products is extremely challenging. The number of drug launches is rising every year, taking competition levels higher with them. And because these novel products tend to be more tightly targeted to smaller patient populations than the “blockbuster” drugs of old, their pricing/reimbursement terms need to be tailored to match. This is especially the case with highly complex biologic drugs, which typically are expensive to research and develop. Below I offer a…
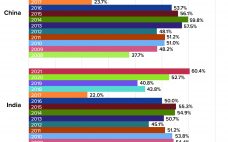
Bioprocessing Facilities in Asia Consider Domestic Alternatives to Western Suppliers
The global biopharmaceutical industry had been growing robustly even before the COVID-19 pandemic. According to the BioPlan Associates Top 1000 Biofacility Index and Biomanufacturers Database (1), bioprocessing capacity worldwide increased an average of 12% over the past decade. China has seen nearly double that rate. India’s bioprocessing segment also is showing strong growth. Because those regions represent 37% of the world’s population — and with rapidly growing middle-class economies, — demand for biologics there is outstripping that elsewhere. Historically, Western…
In Search of a CMO for My Biotechnology Startup: How to Navigate a Journey Without Process Maps
An innovative biopharmaceutical product can transform from an abstract idea at small scale into the basis of a burgeoning startup company. At that point, company leaders seek ways to ensure that a biologic will scale up in a quality-controlled, professional, and sustainable environment. That involves refining a research-stage prototype into a product that will be consistent and reproducible for research and development (R&D) and manufacturing and that will meet all relevant regulatory standards in specified target markets. Within the constraints…
An Ethical Option for Shelved Drugs
A key priority in today’s investment world is corporate adherence to environmental, social, and governance (ESG) requirements. A company’s ESG score serves as a marker of the organization’s values and as a disclosure mechanism for investors to consider. Many companies now consider ESG scores when making strategic choices, and my group has identified a tangible option. In 2019, the Children’s Tumor Foundation and CureSearch for Children’s Cancer, launched the Bridge initiative (1) in partnership with FasterCures, a division of the…
Facilitating Workforce Development: A case study on improving single-use training through vendor and end-user collaboration
Discover how Pall Corporation and Lonza collaborated to improve single-use technology training for operators using a blended approach to learning. This article presents: The importance of SUT training for operators. Why a blended approach ensures that operators get the training they need in the format that best suits their learning style. How collaboration between suppliers and biomanufacturers can shorten training program development timelines and increase the quality of training tools. How Pall and Lonza developed a digital training approach together.…
Patent Thickets Constrain US Biosimilars Market
Biosimilars represent a significant cost-savings opportunity in the United States because they can introduce competition for some of the most expensive and widely used prescription drugs on the market: originator biologics. Several market and regulatory barriers have slowed biosimilar market entry and uptake in the United States (1). Some hurdles are unintentional or transitory; others are deliberately crafted by manufacturers of reference biologics to thwart competition. Among the most significant strategic barriers to biosimilars’ entry into the US market are…
The Biosimilars Action Plan: Promoting Faster and More Extensive Adoption of Biosimilar Drugs
The pace with which biosimilar drugs have been adopted in the United States has frustrated (and displeased) policymakers (1). After passage of the Biologics Price Competition and Innovation Act (BPCIA) (2) as part of the Affordable Care Act of 2010 (3), policymakers intended and expected significant reductions in expenditures for this class of biopharmaceuticals (4). The Federal Trade Commission (FTC) had predicted that the percentage of savings would be lower than that of the <90% reduction in costs for small-molecule…