Biomanufacturers seeking the best approach to rapid implementation of flexible manufacturing capacity take into account the benefits presented by different modular construction options. We analyzed different approaches to building manufacturing capacity and assessed the economic benefits of each approach. Our evaluation was based on biopharmaceutical products for which there is an immediate unmet need, such as treatments or vaccinations for COVID-19. Such products also might entail a sudden increase in demand (e.g., expansion of a product indication or sales ramp…
Business
Benefits of Single-Use Standardization: Adopting a Standard Design Approach
It is widely accepted that standardization of single-use designs and assemblies would be beneficial to the biopharmaceutical industry, providing it quickly with simple and economical solutions. Meanwhile, as implementation of single-use technology increases across the biopharmaceutical industry, suppliers are struggling to keep up with demand. That has been evident particularly in current supply issues caused by the COVID-19 pandemic. A widely adopted single-use standardization approach could help alleviate such supply issues. That would not only benefit the industry by helping…
Untapped Potential of Tissue Engineering: The Three Obstacles Holding It Back
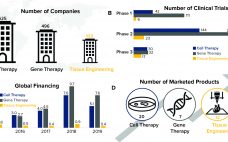
Regenerative medicine is the interdisciplinary field comprising tissue engineering, cell therapy, and gene therapy. These biopharmaceutical modalities, also referred to as advanced therapies, are growing rapidly, characterized by groundbreaking therapeutic advances that have the potential to change how healthcare providers deliver care. As Figure 1 shows, cell and gene therapies have gained traction over the past decade, as evidenced by large increases in investment and the number of marketed products. By contrast, tissue engineering investment and product commercialization has lagged…
The Difficulties of Manufacturing Cell and Gene Therapies At Scale
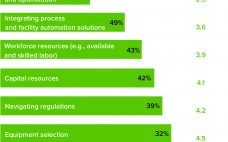
From large-scale manufacturing of one-size-fits-all blockbusters to small-scale processing of personalized therapies, the biopharmaceutical industry has undergone a revolution over the past decade. Among the standout milestones is the development of advanced therapy medicinal products (ATMPs). More than 1,000 of these research-intensive therapies are progressing through clinical trials toward potential commercial manufacturing. Cell and gene therapies (autologous and allogeneic) are targeted for many incurable diseases and conditions, including autoimmune disorders and cancers. Despite the excitement about ATMP potential, developers and…
Manufacture and Regulation of Cell, Gene, and Tissue Therapies: Part 2 — Regulatory Guidances
The US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the Medicines and Healthcare Products Regulatory Agency (MHRA), and Japan’s Pharmaceutical and Medical Devices Agency (PMDA) all offer support and guidance for developers of advanced therapy medicinal products (ATMPs). Some agencies have issued guidelines to help companies through different stages of product development — from research and development to marketing authorization and postauthorization activities. Such guidelines are updated regularly as more knowledge becomes available from the development and…
Trade-Secret Vulnerabilities: Recent Hacking Schemes Highlight the Need to Protect Proprietary Pharmaceutical Information
On 21 July 2020, the US Attorney’s Office in Spokane, WA, unveiled a sweeping indictment accusing a pair of Chinese hackers of conspiring to steal trade secrets from a number of American pharmaceutical companies and research institutions, including biotechnology companies working on potential COVID-19 vaccines (1). Although the hackers apparently did not succeed in their attempt to obtain vaccine data, their years-long conspiracy involved other attacks on the pharmaceutical industry — and netted them troves of sensitive commercial information. Among…
Biomanufacturing Workforce Development: Fostering Talent During and After the Pandemic
Beyond the human suffering and economic damage caused by COVID-19, one of the most powerful results of the pandemic has been to focus global attention on drug and vaccine development for infectious diseases. Massive investments by governments, institutions, and biopharmaceutical companies have accelerated development of novel therapeutics, including messenger RNA (mRNA) and viral-vector vaccines, that are poised to become transformational platform technologies for biopharmaceutical manufacturing (1). Furthermore, the well-established technology of monoclonal antibodies (MAbs) has lived up to its promise…
Emerging Strategies for Drug-Product Comparability and Process Validation: Part 2 — Validation, Legacy Products, and Lifecycle Management
This two-day CASSS CMC Strategy Forum explored many technical, practical, and regulatory facets of biological drug-product (DP) analytics, process validation, and comparability. Part 1 of this report summarized the discussions on drug-product analytics and comparability in BPI’s March 2021 issue (1). Here we report on day two presentations and discussions on validation, legacy products, and lifecycle management. Session Three: Drug-Product Validation The morning session focused on principles of process validation with examples of challenges specific to drug products. New Risk-Based…
Defining a Successful Biotechnology Entrepreneur
Having brought its first vaccine candidates from concept to clinical development in less than three months, BioNTech is making global waves as the pioneer behind Pfizer’s COVID-19 vaccine. Because of such success stories, more eyes than ever are looking at opportunities raised by the biotechnology industry. As talented people around the world from academia, clinical practice, and other industries consider careers in this sector, they should examine the characteristics necessary to become successful biotechnology entrepreneurs. Biotechnology fuses science and business.…
eBook: Buffers — Navigating New Demands on Downstream Raw Materials
Bioreactor titers for monoclonal antibody (MAb) processes have increased significantly since the dawn of the biopharmaceutical industry, yet such gains have instigated bottlenecks for critical high-volume raw materials used in downstream processing, such as buffer solutions. As downstream purification is required for most, if not all, biopharmaceutical products, buffers and their preparation are topics that concern nearly every drug company. But those topics rarely receive direct attention. This BPI eBook explores what factors prompted the current buffer bottleneck and what…