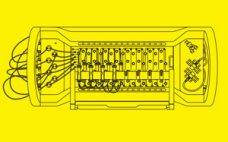
This webinar provides a virtual demonstration of the BioSMB multi-column chromatography platform — a fully scalable single-use solution that reduces resin usage and enables manufacturers to explore continuous processing. The demo starts with an overview of the BioSMB PD system for small-scale manufacturing and then continues with the BioSMB Process systems for clinical and commercial manufacturing. Click here to watch this virtual demonstration.
BPI White Papers
CHO Cell Culture Media & Feeds
Media selection is a crucial step in optimizing and maximizing your upstream process. Our chemically defined catalog media portfolio offers a comprehensive range of off-the-shelf products to support all of your CHO production processes, including fed batch and perfusion mode. Learn more.
Crude Sample Analysis in Process Development: Time and Cost Savings
This overview highlights a few examples from drug discovery and process development where the Octet® system demonstrated a significant reduction in the analysis time over ELISA and HPLC by eliminating the need for purification, while still achieving high accuracy and precision. The total assay times were dramatically reduced with fewer assay steps and less labor time involved. The easy and versatile, fully integrated Octet® assay has additional advantages over other label-free technologies like SPR, where sample washing steps are required.…
Expanding Applications in Downstream Biologics Characterization: Stability, Formulations and Aggregation Studies using Octet®
Structure is an important characteristic for protein activity and function. Structural alterations due to protein mis-folding, denaturation or unfavorable conditions can lead to the formation of aggregate species that can affect the efficacy and safety of biologic drug candidates. While many biophysical analytical tools are well suited for detecting these aggregates, few offer a combination of structural and functions assessment in the same system. Sartorius’ Octet® platform is an easy-to-use analytical tool that can detect the presence of aggregated species…
Transfection Reagents for Cell and Gene Therapy
Polysciences offers high-quality, R&D and cGMP grades of transfection reagents for the smooth transition from process development through clinical trials and commercial manufacturing of viral vectors/protein. Polysciences R&D grade PEI MAX has been used by academic and industry customers for over a decade and is one of the most cited transfected reagents. A few years ago Polysciences launched an easy to use liquid version of PEI MAX which is called Transporter 5 focusing on increased customer convenience. Both PEI MAX…
FAQs: Challenging host cell protein assays for improved risk mitigation
Host cell proteins (HCP) are critical to quality control in biologics development. If left unremoved, they can cause immunogenic responses and reduce drug efficacy. In our last webinar we shared Cytiva’s strategy for designing a comprehensive HCP risk mitigation strategy. We discussed how to challenge your assays early to ensure accurate measurements, build confidence, and reduce the risk of unexpected HCP levels. Our HCP experts highlighted and responded to the most common questions raised around host cell protein assays. Want…
Fragment-Based Screening in Drug Discovery: How to Improve Hit Rates & Deliver Higher-Value Targets
Learn about the basics of fragment-based drug discovery (FBDD), one of the most valuable approaches to small molecule screening and now even more widely used today by pharma companies than high-throughput screening (HTS) HTS’s main limitations — low success rates for more challenging targets, high level of false positives, and the size of the compound libraries — are propelling the use of FBDD. This change is also driven by the rise in the number of explorative targets, which demands not…
Enabling Vaccine Production: Solving Challenges
Vaccine developers are in need of more efficient and cost-effective approaches to manufacturing. To achieve this, we actively collaborate with academia, researchers and manufacturers to develop and optimize innovative tools, processes and strategies to resolve bottlenecks and accelerate the availability of vaccines to the global population. This e-book contains a series of case studies highlighting our recent collaborations with organizations and thought leaders on the front lines of the battle against challenging pathogens. From proof-of-concept to full commercial-scale manufacturing, discover…
Single-Use Systems: Globalizing Best Practices and Technology Specifications
The challenges of multi-national bioprocessing operations are numerous. They include finding highly skilled experts for each site, lack of expertise with single-use systems, contamination risks and redundant efforts and resources—all of which can lead to higher costs. But these challenges also present opportunities for increasing speed to market, eliminating redundant work and overall cost savings. This white paper details the best practices used in global drug manufacturing. The key is global coordination. Best practices and specifications need to be identified…
Keep Your HCPs Under Control
When it comes to manufacturing biologics, managing host cell protein (HCP) levels is essential. A robust HCP strategy can help protect both your timeline and budget. Cytiva understands the challenges researchers face in HCP detection, quantification, and removal. We offer an end-to-end solution for HCP management, and our experts will work with you to find the right strategy tailored to your needs. To get started now, check out our knowledge center for expertise and advice on enhancing your HCP strategy.