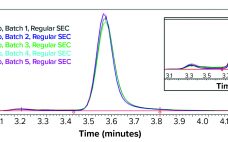
Quantitation of different capsid species in viral-vector gene-delivery drugs, including recombinant adenoassociated virus (AAV) therapies, is essential for proper assessment of critical quality attributes before regulatory approval and during commercial production. If rAAV full-capsid percentages are maximized, and variants such as empty or partially-filled capsids are reduced as much as possible, then potency is increased for improved dosing and tolerance outcomes. Traditionally, characterization of AAV purity with respect to empty, full, and other variants has been performed by techniques such…
Analytical
Quality Risk Management for Filter Integrity Testing: Compliance with EMA’s Future Annex 1
Quality risk management (QRM) is a systematic process for assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. Although QRM is not new (1), the regulatory focus on QRM will increase with the arrival of the European Medicines Agency’s (EMA’s) Annex 1 (2), which was reviewed by the US Food and Drug Administration (FDA), the World Health Organization (WHO), and the Pharmaceutical Inspection Convention Scheme (PIC/S). Integrity testing of sterilizing-grade filters is…
Endotoxin Questions and Answers
Endotoxin — also known as lipopolysaccharide (LPS) — is a large molecule made up of a lipid and a polysaccharide portion found in the outer membrane of each Gram-negative bacterial cell. Examination of endotoxins is necessary for microbial and animal cell-culture operations that produce active pharmaceutical ingredients (APIs) and vaccines. If a cell culture is contaminated with endotoxins, it could affect cell growth and function (thus potentially affecting product quality). Biopharmaceutical companies need to understand the endotoxin levels within their…
Optimizing Cell Line Development for High-Quality Biologics
For a host-cell system to generate high yields of recombinant proteins and other entities, cells must be derived from optimized and stable cell lines. However, cell line development (CLD) can be tedious and time-consuming work, and every stage in the CLD workflow has its limitations and challenges. Researchers are creating advanced strategies and tools to overcome those challenges, especially for complex biologics such as bispecific antibodies (BsAbs) and difficult-to-express (DTE) proteins. Online presentations from the CLD track of the BioProcess…
Use of CRISPR and Other Gene-Editing Tools in Cell Line Development and Engineering
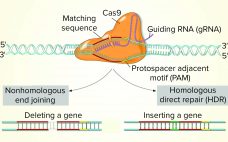
While the role of biologics in treating human diseases has evolved dramatically over the past decade, so has genetic engineering. Rational genetic engineering to enhance biotherapeutic proteins has become a reality catalyzed by publication of the genome sequences of multiple Chinese hamster ovary (CHO) cell lines. Novel “designer” CHO cells modulate posttranslational modifications (PTMs) of recombinant proteins by genome editing, and it is now possible to knock-in or knock-out genes of yeast and mammalian cells precisely (within one DNA base…
Plant-Cell Cultures and Cell Lines for Recombinant Protein Expression
Cell cultures derived from mammalian and bacterial cell lines are the conventional production systems in bioprocessing. But they also have their limitations. Media for mammalian cultures in particular are notoriously expensive, and traditional cell cultures can be highly sensitive to growing conditions. During the late 1980s and into the 1990s, plants and plant-derived cell cultures were introduced as alternative cell-culture systems (1, 2). Although transgenic plants (genetically modified) once looked promising in the early 2000s, the cost and manufacturing complexity…
Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization
Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin G (IgG) product integrity in real time during a bioreactor run, especially…
Development of Patient-Focused Commercial Specifications: Understanding Clinical Relevance and Criticality of Quality Attributes
The CASSS chemistry, manufacturing, and controls (CMC) Strategy Forum on 23 January 2019 in Washington, DC, was entitled, “The Development of Patient-Focused Commercial Specifications Through Understanding of Clinical Relevance and Criticality of Quality Attributes.” This forum covered the definition, identification, control, and management of patient-focused attributes throughout the life cycle (from discovery through approval) of biological products, including vaccines. Participants investigated how to differentiate through the product development life cycle which attributes are “clinically meaningful” from those applied for manufacturing…
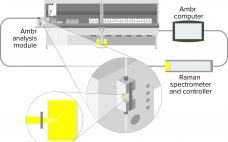
Novel Integrated Raman Spectroscopy Technology for Minibioreactors: Accelerating Raman Model Building for Cell Culture Monitoring and Control
Raman spectroscopy is used widely in biomanufacturing as a process analytical technology (PAT) for monitoring analytes such as glucose and lactate (1). Predictive Raman models also can be used to control glucose concentration in cell cultures (2). The technique is becoming more popular for pilot- and manufacturing-scale bioreactors, but it only recently has been studied with minibioreactors for measuring analytes and producing predictive Raman models for feedback control (3) thanks to advances in integrated technology for automating sampling, analysis, and…
Ask the Expert: An Innovative System for Advanced, High-Throughput Aggregate Analysis
Distinguishing aggregated active pharmaceutical ingredients (APIs) from other particle types is critical to evaluating a protein product’s stability, but standard characterization tools struggle to discriminate proteins from nonproteins with similar sizes, shapes, and morphologies. In a 25 June 2020 “Ask the Expert” webcast, Bernardo Cordovez (founder and chief scientific officer of Halo Labs) introduced his company’s Aura subvisible-particle analyzer. He explained how the device combines an innovative microscopy technique with sophisticated imaging software to characterize subvisible particles more quickly and…