Chinese hamster ovary (CHO) cells have emerged as a robust platform for bioprocessing serving both early and late-stage biotherapeutic drug supply. However, these cells and other hosts (e.g., HEK293), can be optimized for even greater potential through advanced gene editing. For example, when the endogenous glutamine synthetase (GS) gene is knocked out in CHO cells, a sixfold increase in high-producing cell lines is achieved (1). In another study, CHO with annexin A2 (ANXA2) and cathepsin gene (CTSD) knockouts were introduced…
Analytical
eBook: Sensor Technologies — Essential Tools for Bioprocessing 4.0
Sensors are essential devices that can be used for most, if not all, typical biopharmaceutical development and manufacturing processes to monitor fundamental process parameters such as flow, temperature, pH, and dissolved oxygen throughout all process stages. As the bioindustry progresses toward automation, digitalization, and other “Manufacturing 4.0” concepts, robust single-use and smart sensors for bioprocess monitoring will be needed. Read this BPI eBook to garner valuable perspectives on both of these types of sensors. Discussions herein focus on smart sensor…
Evaluating Biosimilars: A View from the Small-Molecule World
For many years the pharmaceutical industry was dominated by small (usually synthetic) molecules, mixed with a number of nonactive materials and encapsulated or (in the really old days) rolled into pills or pressed into tablets. Although synthesizing the active pharmaceutical ingredients (APIs), formulating the dosage forms, and analyzing the materials at every stage of a product life cycle were not always trivial activities, they were relatively straightforward. Most of the tools needed for analyzing/controlling each step of the manufacturing process…
Bioassay Evolution: Finding Best Practices for Biopharmaceutical Quality Systems
Bioassays help drug developers determine the biological activity (potency) of their products, which has been a biopharmaceutical critical quality attribute (CQA) since long before that concept had a name. Because of their complex nature, bioassays are among of the most challenging experiments to perform reliably with dependably accurate results. Consistent assay performance requires a controlled environment and qualified reagents; skilled analysts who understand cell physiology, regulatory requirements, and the latest techniques; and assay protocols that are intelligently developed, characterized, and…
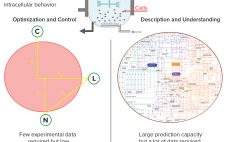
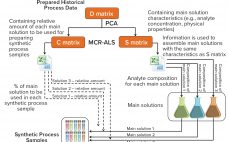
From Big Data to Precise Understanding: The Quest for Meaningful Information
High-throughput technologies have transformed the biotechnology industry. The amount of data they generate is at least a hundred times higher now than it was two decades ago, primarily because of the rise of “-omic” technologies. As in many other industries, the biopharmaceutical sector entered the era of big data the day that high-throughput analytics were routinely implemented in experimental research. Big data refers to “datasets with sizes beyond the ability of commonly used software tools to capture, curate, manage, and…
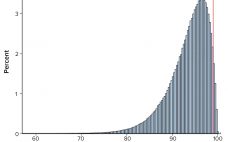
Biopharmaceutical Product Specification Limits and Autocorrelated Data
Calculations, including statistical tolerance intervals, can assist in the development and revision of specification acceptance criteria. Manufacturing results for attributes of a biopharmaceutical product can be positively autocorrelated. The sample standard deviation — calculated from limited, positively autocorrelated data — tends to underestimate the long-term process standard deviation (1). In this article, simulated data are used to assess the relative performance of statistical tolerance intervals, intervals calculated using the minimum process performance index Ppk approach, and the sample range. Prevalence…
Bioprocess Development and Qualification: PAT-Based Stage 1 and 2 Acceleration Strategies
Well-established process analytical technology (PAT) strategies, such as those based on spectroscopy, bring with them several challenges related to the nature of those tools themselves (1–3). Such tools are multiparametric by design — in the sense that most spectroscopies capture multiple attributes sometimes different in nature (e.g., near-infrared, NIR, captures chemical and physical attributes simultaneously). Often a reference method is required; at other times, indirect calibrations are based on the correlation of one culture attribute with another for which a…
Ask the Expert: Highly Sensitive Host-Cell Protein Analyses Using Novel Chromatography Technology
Geert Van Raemdonck (global field support expert at PharmaFluidics) and Koen Sandra (scientific director of the Research Institution for Chromatography, RIC) teamed up for a 10 October 2019 “Ask the Expert” webinar to introduce micro Pillar Array Column (ÎĽPAC™) technology for liquid chromatography–mass spectrometry (LC–MS) for host-cell protein (HCP) detection. Van Raemdonck explained that ÎĽPAC technology approaches chromatography differently than does packed-bed technology. Microfluidic channels with arrays of free-standing pillars are etched lithographically into a silicon wafer. The resulting permeability…
Ask the Expert: Accelerating Timelines By Integrating Cell-Line Development and Manufacturing
In a 31 October 2019 “Ask the Expert” presentation, Nicole Wakes (group leader of Abzena’s cell-line development team) observed that drug sponsors often outsource their early upstream activities to a few different contract research organizations (CROs). But that strategy can thwart short timelines and introduce regulatory and financial risks. Wakes described Abzena’s upstream approach, illustrating how partnering with a single, multicompetent CRO from cell line construction through manufacture can streamline workflows. Integrating cell line development and manufacturing in this way…
Ask the Expert: Developing Bioprocesses for Clinical Manufacturing Success
Biopharmaceutical companies need to make critical chemistry, manufacturing, and controls (CMC) decisions during clinical development of recombinant protein biologics and advanced therapies. In a 17 December 2019 “Ask the Expert” webinar, Nigel Shipston (director of program design at FUJIFILM Diosynth Biotechnologies, FDB) reviewed key aspects of selecting and working with a contract development and manufacturing organization (CDMO). He also highlighted important factors that should be considered during early stages of process development. Shipston’s Presentation The sheer magnitude of investment required…