Filtration of protein-based biologics is essential for minimizing viral contamination and ensuring product safety and high quality. The tendency of therapeutic monoclonal antibodies (MAbs) and recombinant proteins to aggregate under a number of conditions can complicate selection of a virus filter. An increasing demand for high concentration formulations creates additional challenges. When performing filterability studies and to ensure meaningful virus filter evaluations, downstream process scientists must address factors that can lead to aggregation. This special report on virus filtration by…
2022
eBook: Intensifying Processes for Monoclonal Antibodies
The commercial manufacturing success of monoclonal antibodies (MAbs) has become a touchstone of the biopharmaceutical industry. MAbs are so well established that they often are referred to as “traditional” biologics, and well-known MAb processing methods have become a model for processing of other “advanced” or “emerging” therapies. But MAb processing continues to advance as biomanufacturers seek ways to improve efficiencies, lower costs, and (most recently) increase sustainability of facilities. Drug makers are particularly interested in strategies for MAb process intensification.…
April 2022: From the Editor
There’s a funny meme going round social media about the naivete of conspiracy theorists: “My gut is that most of them have never been project managers. Their optimism is adorable.” And the adage may be that “too many cooks spoil the broth,” but I think it’s more like “too many stakeholders can overcomplicate anything.” That can apply to small teams working on relatively simple projects (a group of editors working on a single BPI issue, for example) as much as…
Hurdles Ahead for Cell and Gene Therapy Makers
Significant growth of the cell and gene therapy (CGT) pipeline in recent years demonstrates the enormous potential of these modalities to treat or even cure otherwise intractable diseases. Several CGT products have been approved for clinical use over the past five years. More than 75 such products have come to the market around the world so far. They include chimeric antigen receptor (CAR) T-cell therapies that involve genetic engineering of patient cells ex vivo as well as in vivo gene therapies…
Scalability in Cell and Gene Therapy Facilities: How Today’s Developers Are Preparing for Tomorrow’s Commercial Success
Propelled by year after year of record-setting investments and regulatory approvals, cell and gene therapy (CGT) innovators are on track to revolutionize medicine by providing potential cures for many conditions. Now, CGT manufacturers must plan for a future beyond the production constraints of laboratories. What needs to happen now to prepare for a sustainable, commercially viable scale-up process in years to come? To answer that and other important questions about CGT production, my company, the CRB Group, surveyed more than…
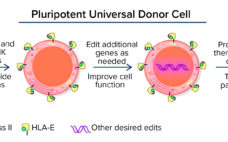
Novel Technologies for Advancing Allogeneic Cell Therapies
In many ways, cells are the ultimate therapeutic product. They can integrate and participate in different biologic processes and replace missing biological functions. Cell therapies are dynamic, versatile, and with the appropriate engineering, capable of influencing and correcting disease processes robustly. Cell therapies essentially are living medicines, and their adaptability contrasts with conventional drug modalities that generally have only a single specific target or effect. Because cell therapies are highly complex modalities, their scientific and R&D challenges are different from…
Process Intelligence: Gene Therapy Case Study Shows That the Journey to Improved Capabilities Starts with One Step
The product development team at a gene therapy contract development and manufacturing organization (CDMO) was working on a high-priority drug-substance project for a key client. The material was crucial to that client’s early stage clinical trial, with an immediate value over US$500,000 to both the client and the CDMO. Unfortunately, the bioreactor used in the upstream process — a transfection unit operation for an adenoassociated virus (AAV) vector — had developed an intermittent problem that could force it to shut…
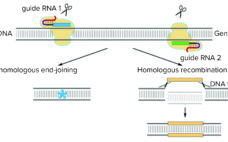
The CRISPR Saga (So Far)
Since January 2016 (with a brief interlude as described below), the Patent Trial and Appeal Board has been attempting to adjudicate the proper inventorship of CRISPR technology in (to date) six separate patent-interference proceedings. (Scientific “priority has been decided, for now, by the awarding of the Nobel Prize in Chemistry to Jennifer Doudna and Emmanuelle Charpentier in 2020; see the “Priority Claims” box). CRISPR-based gene editing was hailed as the “Breakthrough of the Year” in 2015 (1), and the scientific…
Partnerships Are Crucial to Getting Advanced Treatments to Market
New treatments and approaches for tackling serious diseases are being developed using cell and gene therapy (CGT). CGTs are adding a new dimension to the way patients with such conditions can be treated in modern healthcare. But these advanced treatments require complex research, development, and manufacturing processes. CGTs are related and to a degree interchangeable. However, gene therapies typically use some kind of genetic delivery system, (e.g., virus-based vectors) to treat a disease by replacing a malfunctioning gene or introducing…
Advancing Logistics for ATMP Manufacturing
Advanced therapy medicinal products (ATMPs) hold much potential for improving healthcare. They offer hope for treating or even curing patients. The biopharmaceutical industry has recognized the importance of making such therapies accessible to as many people as possible. To provide personalized ATMPs, biomanufacturers are shifting toward flexible, patient-centered production processes. A Paradigm Shift in ATMP Manufacturing Ex vivo cell and gene therapies are particularly promising approaches to personalized regenerative medicine. Thus, it is no surprise that the numbers of US…