Richter-Helm is a Hamburg, Germany–based contract manufacturing company with a proven 30-year track record and specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines. Our seasoned, 240-strong team supports you with process development, supply of products for clinical trials,…
Industry Innovators 2019-2020
Rapid Process Optimization with Customizable Single-Use Bioreactors: The Bioreactor That Adapts to Your Bioprocess
To enhance their performance, life science specialists require flexibility in their work and tools. The increasing diversity of bioprocess applications calls for different cultivation methods. Therefore, being able to customize processes and equipment is a significant advantage. With over 45 years of experience in customizable multi-use bioreactors, Applikon Biotechnology now provides the same flexibility with customizable single-use bioreactors. The AppliFlex ST bioreactor is a fully configurable, stirred-tank, single-use bioreactor that can be designed to specific customer demands. Using three-dimensional (3D)…
Advancing Robust Manufacture of T-Cell Therapies: Application of Single-Use Stir-Tank Bioreactors
With the increase of T-cell immunotherapy research, cell therapy developers have encountered the difficulty of producing consistent, high-quality products. Approved therapies Kymriah™ (tisagenlecleucel, Novartis), Yescarta™ (axicabtagene ciloleucel, Kite Pharma), and late-phase clinical products BB2121 and UCART19 have been critiqued for presenting potential obstacles to patient access because of high costs of treatment and variable manufacturing outcomes. Manufacturing processes need to be developed to increase control over each unit step in a way that improves product consistency over the life of…
Eppendorf BioBLU® 10c Single-Use Vessels Simplify Handling of Cell Culture Bioprocesses at Bench Scale
Substituting traditional glass bioreactors with single-use equipment can simplify a bioprocess workflow. Implementing single-use vessels eliminates the need for cleaning and autoclaving, thus reducing the time needed to prepare a bioprocess run and lowering contamination risk. Another advantage is that single-use vessels reduce occupational hazards associated with overweight handling (especially at larger bench scales) because plastic weighs less than glass. Fill out the form below to read the complete white paper now.
Updating Biologics Manufacturing: Using Modern, Single-Use Powder Handling Reduces Time to Market and the Chance of Contamination
Biopharmaceutical companies are striving to produce biologics more efficiently with a lower cost point and fewer employees. Most small-scale media and buffer operations are carried out with small bottles of liquid media. But as processes are scaled up to manufacturing levels, that is no longer a viable option. Costs of shipping liquid are simply prohibitive, so the industry has migrated to using media and buffers in powder form. In fact, 90% of cell culture media and buffers for sale is…
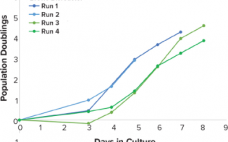
Increase Viable Sf9 Cell Density and Baculovirus-Based VLP Production by Supplementation with Recombinant Insulin Human AF
Virus-like particles (VLPs) have become a promising means for developing vaccines and gene therapies. Currently, vaccines based on VLPs are commercially available for human papillomavirus and hepatitis B and E. Other disease treatments with VLPs are undergoing clinical trials. Upon expression, the structural matrix polyprotein group-specific antigen (gag polyprotein) from the human immunodeficiency virus (HIV) has shown to accumulate beneath the lipidic membrane. After a sufficient number of gag polyproteins are recruited, the assembly process is finished, and the VLP…
New Single-Use Bioreactor for Cellular Products of Consistent Quality: BIOSTAT® RM TX Bioreactor with Flexsafe® RM TX Bags
Sartorius Stedim Biotech (SSB), a leading international supplier for the biopharmaceutical industry, has launched the BIOSTAT® RM TX single-use bioreactor, a new wave-action mixer system developed specifically for closed, automated expansion of consistent-quality cell therapies such as ex vivo cellular immunotherapies. This new good manufacturing practice (GMP) platform combines SSB’s established single-use Flexsafe® bag technology with the company’s expertise in biopharmaceutical automation. Fill out the form below to read the complete technology review.
Cell Culture Expansion in Fully Closed Erlenmeyer Shake Flasks Outside the Biosafety Cabinet
Expansion of suspension cell culture from cell bank to bioreactor is performed through passages of successively larger Erlenmeyer shake flasks. A traditional cap must be unscrewed for each fluid transfer. Risk of contamination is mitigated by performing these fluid transfers in a biosafety cabinet (BSC) or laminar flow hood. Work in a BSC is not preferred because of high maintenance and operating costs, intensive cleaning and decontamination procedures, and the inconvenience of performing operations in the BSC. Despite the use…
New Approach for Qualifying Liquid Handling in Single-Use Bags
2D Flexsafe® Bags and Shells Offer Strong Validation: Complete logistical solution for handling and shipping of liquid bulk drug substance in a 2D Flexsafe® bag & shell Quality by design principles Comprehensive and innovative testing program following the guidelines like ASTM, NF and ISO The system allows for safe variable shipping volumes of 10–120% of nominal fill volume and can be leveraged by the end-users for their own process validation Fill out the form below to view the complete poster…
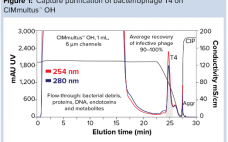
Platform Purification of Clinical-Quality Bacteriophages
Antibiotics are unable to keep pace with infectious diseases, and the use of bacteriophages is a timely solution to that problem. But implementation of bacteriophages involves overcoming some challenges. Broad-spectrum clinical efficacy will require numerous phages. In turn, cost-effective development of purification procedures will require a platform approach in which one basic process can accommodate all species. That includes the ability to reduce endotoxins to appropriately low concentrations despite the high loads that occur in gram-negative production systems. Monolithic chromatography…