Even in “normal” times, companies need to balance time to filing an investigational new drug (IND) application against careful consideration of processes that can have far-reaching consequences on the quality of biologic products. But supply-chain interruptions still feature prominently in the news during the ongoing COVID-19 pandemic. From equipment to chemicals to plastic components, end users, their suppliers, and (critically) their suppliers’ suppliers all are feeling growing uncertainty about production timelines and availability of materials. In this eBook, BPI’s editor…
Thursday, October 28, 2021 Daily Archives
October 2021: From the Editor
With autumn beginning to assert itself, our days here in Oregon are cooler, and long-awaited rain is coming. From drought and wildfires to tropical storms and flooding around the globe, we hope that you and your loved ones are making it through this year’s extreme weather events unscathed. And we continue to wish you well through the continuing healthcare crisis. Autumn is time for the annual BPI Conference (including its parallel cell and gene therapy track), held as a hybrid…
Collaboration Agreements: Critical Issues and Common Pitfalls
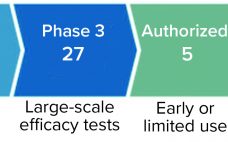
The number of collaboration arrangements for emerging technologies is increasing significantly, especially among companies developing COVID-19 diagnostics, vaccines, and therapeutics. Consequently, a massive amount of capital was invested in the biopharmaceutical industry in 2020. These deals range in value from the low millions to several billion dollars, representing significant risks and value for investors. Some recipients of that capital are seeking partners with whom to collaborate, sharing both the costs and risks associated with their activities and bringing innovative technologies…
Implementation of Established Conditions: Learnings from a “Sharing Science Solutions” Workshop
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline Q12 (1) (step 4 sign-off in November 2019) is in the process of being implemented in a number of regulatory regions. The document provides additional frameworks for pharmaceutical life-cycle management. It is intended to support globally harmonized regulatory tools such as established conditions (ECs) and product life cycle management (PLCM) documents to facilitate postapproval changes to chemistry, manufacturing, and controls (CMC). Although a harmonized framework…
Intellectual Property and COVID-19
The COVID-19 pandemic is the greatest global health threat caused by a virus since the influenza pandemic of 1918 (and, before that, innumerable smallpox outbreaks throughout history). So far, the number of infections and deaths has not reached levels seen during the “Spanish flu” pandemic. However, travel, global trade, and modern factors such as misinformation on social media have increased infection rates and risks of infection. As of 15 July 2021, the World Health Organization (WHO) had reported 188,655,968 confirmed…
Designing Vaccines: The Role of Artificial Intelligence and Digital Health, Part 1
According to the founder and executive chairman of the World Economic Forum, Klaus Schwab, the fourth industrial revolution began in the 21st century and is characterized by an unprecedented development and exponential growth of a high-technology industry transforming society at every level (1–4). In particular, healthcare systems are evolving rapidly to adapt to the new reality. According to Forbes, the main technologies currently shifting the paradigm of medical research are artificial intelligence (AI) and machine learning (ML) (5), both defined…
MAb Viral Clearance Studies:
A Substantiated Platform Approach for the IND Stage
Virus removal/inactivation is a major concern in the safety of monoclonal antibodies (MAbs) and other recombinant-protein drugs. Some methods (such as nanofiltration and low-pH inactivation) have been demonstrated repeatedly by the industry to be reliable for most viruses, with >4 log10 removal. Based on my company’s virus-removal experiences with its MAb downstream-process platform, we propose a “bracketing method” — testing only samples that lie at the extremes of a design space — to prove proactively that small differences in operating…
Cell Media Analysis: A Critical Piece of the Bioprocessing Puzzle
This webcast features: Graziella Piras, Bioprocess Segment Director, 908 Devices Cell culture medium remains a key component of traditional monoclonal antibody (mAb)-based biologics as well as newer modalities such as gene and cell therapies manufacturing. As such, cell culture media development and optimization continue to be an important focus for the biopharmaceutical industry. Increased analytical capabilities have provided new insights into the relationship between cell culture media, the cells they support, and ultimately the outcome of the final product. That greater insight offers more…
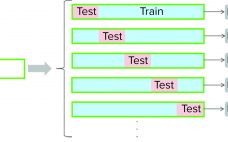
Multivariate Data-Driven Modeling for Continued Process Verification
Continued process verification (CPV) is an integral part of process validation for the manufacture of human and animal drugs and biological products (1). It is designed to meet three primary goals: maintain a validated state of products, their processes, and related systems; enable continuous process improvements; and meet regulatory requirements for life-cycle validation. A CPV program for a biologic product entails regular collection of data related to critical process parameters (CPPs) and critical quality attributes (CQAs) and the preprocessing, analysis,…
Risk Determination of Potential Mycotoxin Exposure to Patients: Testing Recombinant Human Factor VII from Transgenic Rabbits
Sevenfact eptacog beta is a new recombinant human factor VIIa (rFVIIa) developed by LFB SA in Les Ulis, France, as a bypassing agent (BPA) for treatment and control of bleeding in people with hemophilia A and B and inhibitors (1, 2). The product was approved for use in adults and adolescents by the US Food and Drug Administration (FDA) in April 2020 (3). It is expressed in the milk of transgenic rabbits and purified through a multistep process using both…