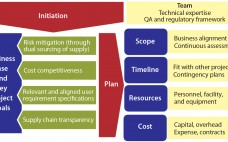
Ensuring a continuous supply of safe medicines is a key objective for the pharmaceutical industry and health authorities alike. A critical component to that end is maintaining a reliable supply of qualified raw materials (RMs) used in drug production. However, changes in suppliers, their processes, their providers, and consequently the materials they supply can occur (for a number of reasons) at any time during the life cycle of drug production. A product-supply organization therefore must be prepared to address such…
Biochemicals/Raw Materials
Comparison of Concentration Measurement Technologies in Bioprocess Solutions
Biopharmaceutical manufacturing involves complex process steps. Exacting production conditions are typically required to maximize the yield, purity, and quality of biological products. In recent years, process analytical technology (PAT) has been increasingly used to monitor key process and performance parameters in real time. That has enabled better control of production conditions. An important parameter required to achieve consistent results in many bioprocessing steps is solute concentration in process fluids. The Critical Need for Concentration Measurement Many biopharmaceutical manufacturing process steps require measuring…
Strategies for Microcarrier Culture Optimization
The process of delivering an allogeneic stem-cell therapy to patients requires isolation and expansion of rare tissue-specific stem cells, which are subsequently delivered to individual patients for treatment. One type of cell used for such therapies is commonly known as human mesenchymal stem cells (hMSCs). They have been isolated from a number of tissues: e.g., bone marrow, heart, brain, placenta, and umbilical cord. And they have been shown to be immune-privileged in that hMSCs elicit no graft-versus-host (GvH) response such…
A Quick Guide for Sourcing Biopharmaceutical Raw Materials
Before the ratification of regulatory guidelines from The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Q8–Q11 (1–4) — whose scope includes raw materials for biopharmaceutical production — many drug manufacturers chose the most cost-effective and readily available raw materials sourcing options without specifically considering the provenance of those materials. Depending on the chosen supply chain, such materials could be of widely varying quality and not necessarily suitable for a destined application. Raw-material…
Consistently Superior Cell Growth: Achieved with New Polyethylene Film Formulation
During the past decade, single-use bioprocessing bags and bioreactors have gained a significant foothold in the biopharmaceutical industry because they offer a number of advantages over traditional stainless steel equipment, especially for clinical production, multiproduct facilities, and emerging economies. At the same time, some companies are concerned that plastic materials might release potentially toxic substances that could affect cell growth and product titers (1). In a worst-case scenario, they could even compromise drug safety when a company uses disposable bags…
Supply Chain Challenges in the Biopharmaceutical Industry: A Case Study Following the 2011 Tsunami in Japan
Global manufacturing of biopharmaceuticals for human use helps save the lives of millions of people and is a large commitment to public health. The industry operates in an environment with financial uncertainties and complex international supply chains, so the question of risk mitigation is paramount. There is an expectation that comprehensive risk mitigation programs should be in place to minimize the risk of supply chain interruptions that would negatively affect the manufacture of these vital therapeutics. Here we share how…
Effective Cryopreservation and Recovery of Human Regulatory T Cells
The list of conditions being targeted by cell therapies is rapidly growing, but commercializing cells for widespread medical use will require standardized laboratory practices. Development processes must be adapted specifically for cell-based drug products. Regulatory T-cell therapy represents a promising new frontier in the immunotherapy of autoimmune disorders, especially for patients who have been refractory to available treatments. Because of intrinsic fragility, cell therapy products can be highly sensitive to variations in manufacturing procedures. Standardization of drug-product cryopreservation and storage…
Powders and Bulk Liquids
The two major bioprocess fluids — culture media for upstream production and buffers for downstream processing — are classic single-use products. They are used once and then disposed of. The two basic options for both differ by physical state: powdered media and buffers (“powders” for in-house preparation of liquids by end users) and bulk liquid culture media and buffers, which are fully prepared by their suppliers (“liquids”). We conducted market research studies comparing the benefits and risks (value…
Activatable Immunoconjugates for Target Cancer-Cell–Specific Diagnosis and Therapy
In cancer treatment, early diagnosis and targeted therapies are assumed to yield the highest cure rates. However, most current methods are limited by their low sensitivity to early disease and a lack of specificity for targeted cell killing. Newly developed, activatable immunoconjugates assist in the accurate detection of cancer through in vivo imaging with high target-to-background contrast (1,2). They also provide for the possibility of highly specific, light-mediated treatment with minimal effects on healthy cells surrounding tumors (3). In fact,…
IgM Purification with Hydroxyapatite
Hydroxyapatite (HA) has a long and successful history in the field of antibody purification, and it has worked well for immunoglobulin M (IgM) monoclonal antibodies (MAbs) (1,2,3,4,5,6,7,8). Applications range from initial capture to intermediate purification to final polishing. HA is best known for its superior ability to reduce antibody aggregates, but it also supports excellent reduction of DNA, viruses, and endotoxins. As IgM MAbs exhibit increasing potential in the fields of cancer and infectious disease and in stem-cell therapies, HA’s…