Digital transformation is at the heart of many biopharmaceutical companies’ strategies for ongoing success. However, the definition of digital transformation and what it consists of differ within the bioprocess industry (and might even vary within a single company), specifically as it applies to the overall value chain from R&D to clinical trials, manufacturing, supply chain, and eventually commercial operations. To provide a perspective on what digital transformation could look like in bioprocessing, we present a case study about an exploratory…
Manufacturing
Automated Process Control Based on In Situ Measured Glucose Concentration
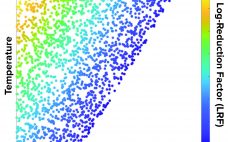
A process analytical technology (PAT) strategy involves defining critical process parameters (CPPs) of a biomanufacturing process that influence critical quality attributes (CQAs) and controlling those CPPs within defined limits. Doing that enables consistent product quality and helps companies reduce waste and costs. Glucose is an important CPP in bioprocessing and cell therapy. Glucose often is fed as a bolus addition based on daily off-line measurements, but that can lead to high glucose fluctuations and to excessive glucose feeding, which can…
Realization of Quality By Design and Beyond: The Intelligent Cell Processing System
Cells are used as raw materials, intermediates, and final products in biopharmaceutical and cell therapy manufacturing. Because living cells are always in a dynamic state, their characteristics must be kept within specified ranges throughout bioprocessing to preserve their utility. Cell population expansion without changing the original cell properties is key for obtaining the required number of cells at the next step. However, limited process data are collected during the expansion phase — information that could be used to understand and…
Get High-Throughput, Definitive Identification of Viable Cells and More in Your Cell Therapy
Analyzing for viable cells using traditional methods such as flow cytometry often encounters clogging resulting in the loss of precious cell therapy samples. Not only is it low throughput, but incredibly complicated, which can lead researchers to misidentify cellular and non-cellular material and confuse cell viability results with product-purity issues. Additionally, it is a regulatory requirement for all injectable drug products be characterized for sub visible particles (SVP) and aggregates that may form during a manufacturing process. With Backgrounded Membrane…
Addressing Critical Issues in Cell and Gene Therapy Manufacturing
Recombinant lentivirus (LV) and adeno-associated virus (AAV) are critical components of cell and gene therapies, which show great promise for treatment of diseases from genetic disorders to cancer. Accordingly, there is an unprecedented need for high titer and large-scale viral vector manufacturing processes to support the growing number of researchers developing biotherapeutics for immunotherapy. Download this Special Report, highlighting: Evolutions of gene therapy manufacturing Improvements to GMP raw-material sourcing. Role of transfection reagents like VirusGEN® GMP as a turnkey element…
The Green Imperative: Part 3 — Postuse Management of Single-Use Bioprocessing Materials, Today and Tomorrow
The world desires a more sustainable economy in which resources can be saved, products can be profitably used, and at the end of their useful life, component materials can be recycled into other useful products. The bioprocessing industry has made efforts to meet those goals and has learned a great deal about the role of plastic components in sustainable manufacturing. The most important lesson might be that a science-based approach is required to provide an accurate benchmark of manufacturingʼs environmental…
The Biosimilars Action Plan: Promoting Faster and More Extensive Adoption of Biosimilar Drugs
The pace with which biosimilar drugs have been adopted in the United States has frustrated (and displeased) policymakers (1). After passage of the Biologics Price Competition and Innovation Act (BPCIA) (2) as part of the Affordable Care Act of 2010 (3), policymakers intended and expected significant reductions in expenditures for this class of biopharmaceuticals (4). The Federal Trade Commission (FTC) had predicted that the percentage of savings would be lower than that of the <90% reduction in costs for small-molecule…
Nonideal Colligative Properties in High-Concentration MAb Solutions
Injectable-drug formulations for both subcutaneous and intravenous administration are designed to be consistent with the number of solutes present in human tissue. Such consistency with physiological conditions is achieved by adding an appropriate amount of salt and/or sugar to attain the desired tonicity. Care must be taken to prevent exposure of cells to either hypotonic or hypertonic formulations that could cause lysis or shrinking, respectively (1). Injection of formulations that deviate from human-plasma osmolality (295 mOsm) can cause pain upon…
Designing Vaccines: The Role of Artificial Intelligence and Digital Health, Part 2
In BPI’s October 2021 issue, part one of this review introduced the concepts of machine learning (ML) and artificial intelligence (AI), identifying some broad areas of application within vaccine discovery, preclinical testing, and clinical studies. This month, we conclude with a detailed discussion of specific disease targets and AI’s potential in addressing them. Having highlighted the example of Zika virus in part one, below we focus on malaria, tuberculosis, human immunodeficiency virus (HIV), herpesvirus, hepatitis, and pandemic coronavirus. Malaria Malaria…
Formulation, Fill and Finish of Lentiviral Vectors Part 2: Key Decisions and Risk Management
Over the past few years, Oxford Biomedica UK has developed and implemented its fill–finish platform at its 84,000-ft2 “Oxbox” manufacturing facility constructed in 2019. The first phase of development (45,000 ft2) houses four segregated suites for producing bulk viral-vector drug substance (VS) where closed systems and bioburden-control processes apply, and two fill–finish suites for viral-vector drug product (VP) in aseptic processing. The first of the fill and finish suites is expected to be approved in the first half of 2022.…