The world desires a more sustainable economy in which resources can be saved, products can be profitably used, and at the end of their useful life, component materials can be recycled into other useful products. The bioprocessing industry has made efforts to meet those goals and has learned a great deal about the role of plastic components in sustainable manufacturing. The most important lesson might be that a science-based approach is required to provide an accurate benchmark of manufacturingĘĽs environmental burdens. Life cycle assessment (LCA) has revealed that, in general, processes using single-use technologies (SUTs) often have smaller environmental footprints than processes based on durable systems (1, 2).
A proper final assessment of either intermediate values or strategic conclusions would include consideration of uncertainty factors. Researchers can attempt to quantify those factors in a risk assessment by pursuing scenario analyses. Some uncertainty factors can be evaluated by integrating scenario analyses as part of life cycle and quantitative risk assessments, if the probabilities of scenario occurrence (and of other future events and their consequences) are incorporated. Modern modeling approaches can examine all possible pathways as a single product system containing well defined, but uncertain processes. Based on the probability evaluated by a model, individual processes then can be selected to evaluate variability in the targeted outcome. Uncertainty analyses then can provide a probability distribution for each impact score, accounting for all known sources of uncertainty (3).
Circularity is another useful guide in our approach to improve SUTs. That strategy involves polymer selection, plastic-component development, manufacturing, shipping, packaging, and postuse handling. Also, companies can rethink the petroleum basis of plastic resins, reduce the mass of plastic in a single-use product, and/or reduce the number of plastic-dependent activities through process simplification. Biopharmaceutical companies also can reduce the number of components required per unit of product by reengineering an operation either to intensify its productivity or reuse a plastic product in another function.
The first article of this series introduced major themes in sustainability of SUT biomanufacturing and distinguished those products from common disposable consumer items. An LCA comparing traditional and SUT-based manufacturing evaluated multiple options for the postuse handling of SUT materials (1, 2). The analysis showed postuse handling to be a minor component of the worldʼs total environmental burden. Because progress on all fronts is desired, however, the Bio-Process Systems Alliance (BPSA) is examining and cataloging the relative benefit of all available and proposed postuse options to promote their development, consideration, and potential application (1). The second article in this series outlined approaches in the design, shipping, and use of materials in support of the “rethink, reengineer, reduce, reuse, and recycle” paradigm for single-use manufacturing materials (4).
Below, we present the last article in the series on sustainable solutions for SUT bioprocessing. We address current and projected postuse handlings and destinations of plastic materials. The current state of those approaches to reducing environmental burden is quite dynamic. Divergent priorities are offered in published literature regarding the burdens to be considered, the ultimate potential of each proposed remediation, and the current state of those proposals’ development. Although recognized individual powers or identified concerns might be indicated herein, no endorsement or recommendation of any particular approach is intended.
The annual mass of single-use bioprocessing materials is small compared with general packaging and consumer convenience items, whereas the value of pharmaceutical and medical products in contributing to human health and safety is high. On the other hand, many composite materials used in bioproduction such as multilayer polymer laminates can be more difficult to recycle than common homopolymeric resin-based convenience items.
Figure 1 summarizes the (theoretically) available approaches to handling used plastic material. The current practicality of an end-of-life technology for a particular pharmaceutical entity application is a moving target. Factors to consider include the composition and scale of an application’s waste stream and the scalability and current commercial availability of the technology in the application’s geographical setting. Because there is as yet no universal terminology, we started with the classification provided by an official communication by the European Commission (5) and adapted it to cover most technical options. The technologies in Figure 1 can be described further using characteristics such as currently available scale, likelihood of future completion/acceptance, environmental burdens addressed, environmental trade-offs, process economics, and processable feedstocks (e.g., municipal waste and biomanufacturing, healthcare, and other industrial wastes). Those characteristics differ in importance because of a source’s specific process, waste generation, reprocessing locations, and service infrastructure. A glossary is provided on the BPSA sustainability website (6).
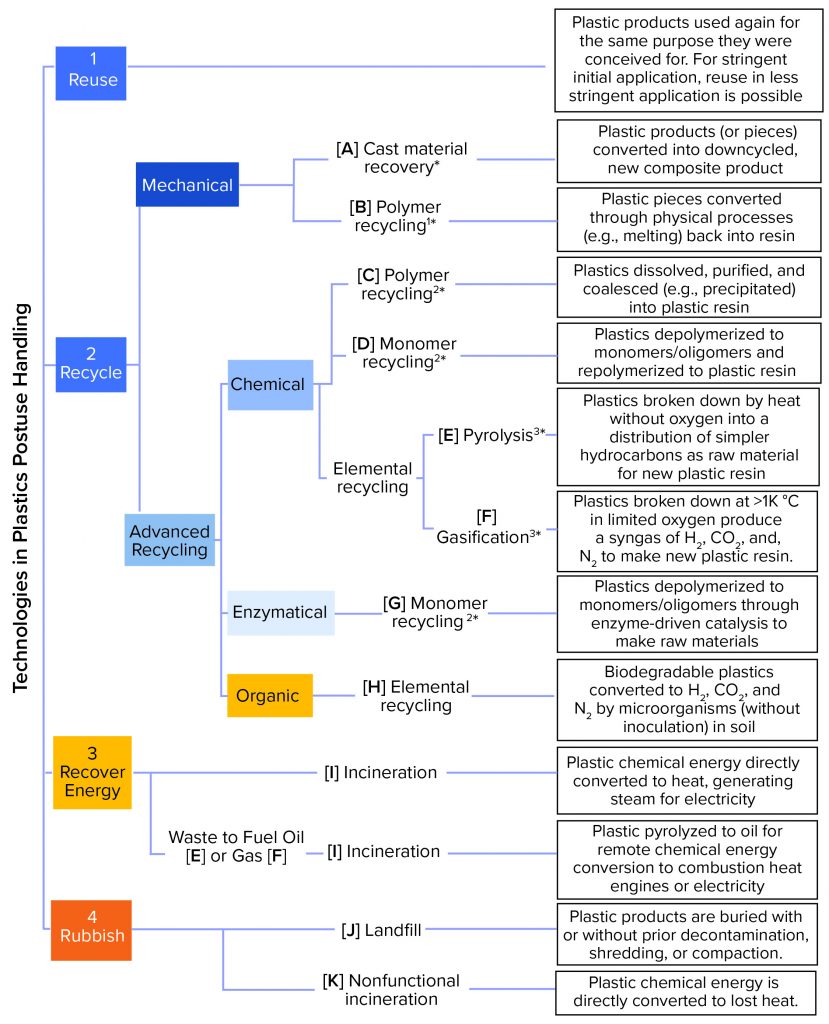
Figure 1: Approaches to handling used plastic; options are ranked according to circular economy recommendations from 1 (most desirable) to 4 (less desirable). Blue boxes indicate that the option is part of a circular economy. Maturity level in the plastics industry is shown in a range of light (less mature) to dark (more mature) colors. Options in orange boxes are out of circular economy. Asterisks (*) represent process energy consumption (not taking into account logistics) as a range of low (*) to high (3*).
Reuse
The best way to reduce plastic waste is to reduce plastic use. Reduction of materials is a design goal that makes environmental and economic sense. Optimization of material use through careful design reduces the need for end-of-life processing. Once a biomanufacturer uses a commercialized plastic component, the most efficient way to reduce its environmental burden is to use that product again (4).
Theoretically, the product might be reused in the same application or a similar operation, or the product might be reused in a less critical application with fewer regulatory or qualification requirements. For example, a purified fluid container might be recovered and used for handling the same fluid before purification. Reusing containers for waste collection is another way to reduce consumption.
Reuse reduces the quantity of plastic waste generated. The net benefit of a reuse strategy is limited if cleaning or other processing is required before reuse.
Recover Energy
Direct energy recovery (Figure 1I), also referred to as waste to energy (WTE), replaces fossil fuels combustion with plastic-waste incineration as a source of usable energy (7) while reducing the volume of material that is landfilled. Although this approach to the elimination of solid waste cogenerates energy, it emits carbon directly into the atmosphere. The amount and number of other pollutants emitted are subject to both a particular facility’s combustion efficiency and its exhaust gas scrubbing. Surveys suggest that currently WTE is the waste management method most widely used by biopharmaceutical manufacturers today (8). WTE can help companies manage contaminated materials, prevent the need for grinding or other preprocessing, reduce transportation costs, and contribute fuel to on-site power cogeneration. WTE prevents undesirable effects such as land burden, chemical leaching into ground water, release of intermediate gases such as methane, ocean contamination, and long-distance transport.
The amount of allowable emissions can differ according to geographic location. In some regions, incineration without cogeneration is unfavorable from economic and social standpoints. Incineration facilities also can be a source of contention for nearby residents, who typically hold a not-in-my-backyard “NIMBY” feeling toward them. No one wants to live near a plant that can host hundreds of trash-filled trucks every day. Usually, the plants end up near low-economic communities (9).
Pyrolytic liquid-fuel generation (Figure 1E) has been used in applications such as coal gasification to produce fuels for municipal lighting and energy. Using the same chemical principles, facilities for the conversion of plastics to fuel-grade liquids and gases have been established. Those facilities differ in scale, input material restrictions (or preferences), process parameters, and the conversion catalysts used.
Plastics pyrolysis is thermal decomposition in the absence of oxygen of hydrocarbon-based polymer molecules into shorter hydrocarbon molecules. Pyrolysis processes can create gas, liquid, or solid products because many different hydrocarbons can be produced. After condensation, the resulting liquid stream can be used as is or separated into different components such as diesel fuel, naphtha, and other petrochemical products. As a plastic-waste management method, pyrolysis can provide the following benefits:
• Potential energy is stored for convenient use.
• Products created are stable, easily transportable, and potentially directly usable.
• Pyrolytic liquid can replace, pound for pound, petroleum pumped from the ground.
Those benefits are offset by the energy required to run the process and the carbon released when pyrolysis products are used as fuels. As with WTE processing, pyrolysis to generate fuels does not create a circular economy. Rather, such waste-management strategies postpone the ultimate demise of the materials and reduce consumption rates of petroleum-based raw materials.
Gasification (Figure 1F) typically involves high heat and a little added oxygen to convert solid materials into a gas (referred to as a syngas) of mostly molecular hydrogen, carbon dioxide, carbon monoxide, and methane.
Incineration (Figure 1I) requires an abundance of oxygen, inducing combustion, which degrades the polymers while producing heat and carbon dioxide.
Although incineration, pyrolysis, and gasification all use high heat, the main difference is in the amount of oxygen supplied during conversion. Pyrolysis heats solid polymers in the absence of oxygen. Gasification is a similar process but requires less oxygen than incineration does. That prevents combustion but supports the production of other reactions and products. Although incineration produces heat but no useful chemicals, both pyrolysis and gasification produce recyclable fuel products or useful reagent chemicals such as naphtha, methanol, and hydrogen. Currently, however, the production of synthetic gas used to fire turbines for heat or electricity is not economically competitive in countries where natural gas is cheap or inexpensive to import and where regulations do not limit its use.
Rubbish
Landfill (Figure 1J) deposition is a common approach to postuse handling (8). Individual applications involve different combinations of size minimization by grinding or shredding, decontamination, and compaction. Landfills can prevent ocean contamination, a product’s immediate carbon evolution, and secondary carbon emissions from long-distance transport. The decomposition of solid waste over time can produce methane-containing biogas, which some municipalities use as an energy source to generate heat or electricity. However, because of the extended timeline (about several decades) for the breakdown of plastics (10), recovery of hydrocarbon gas from a plastics-rich landfill is impractical. Landfills do suffer from land spoiling and the generation of plastic fragments at different scales (macro, micro, nano), which will remain in the environment for a long period. Eventually, bacterial, chemical, and radiation-induced decomposition also will emit different hydrocarbons such as methane and carbon gases into the atmosphere. Moreover, spaces for landfills are becoming limited, with improperly managed rubbish mountains being formed in several developing countries. That has become a source of public consternation, pushing governments to take action and even ban the import and export of plastic waste and other plastic items.
| How Has COVID-19 Accelerated Consumption of Single-Use Plastics in Healthcare?
Plastics have played a major role during the COVID-19 pandemic. They have been used to protect people and accelerate vaccine development and production. The amount of plastic waste generated has increased greatly, especially from personal protective equipment (PPE). Taking into account face masks and shields alone (largely composed of polymeric materials such as polypropylene), the amount of plastic waste generated worldwide since the outbreak is estimated at 3.4 billion units/day (17). With a conservative assumption of 5 g on average per mask–shield mix, 119,000 tons of plastic is wasted weekly. Biopharmaceutical single-use plastics consumption is about 30,000 tons per year, based on a widely quoted 2018 estimate (18). Current consumption is greater than that because the industryʼs growth and increased demand from COVID-related projects. However, even with an extreme estimate of tripled consumption since 2018, biopharmaceutical manufacturing consumes less plastic in a year than is required for COVID PPE in a week. Single-use bags and components for drug production typically are incinerated or sent to landfills (8) in a well controlled waste stream, whereas postuse handling of PPE used by individuals and outside of a regulated environment is not controlled. This comparison of use is offered in support of considering the values and burdens of single-use biomanufacturing materials in the context of other current activities generating plastic waste. |
Nonfunctional incineration (Figure 1k) is the burning of waste to reduce its volume without recovering energy. Nonfunctional incineration is becoming an unacceptable option because the now generally available WTE approach at least uses a resin’s stored chemical energy. Yet modern implementations do provide some benefit. A survey by the European Environment Agency in 2016 (11) found that the top recycling countries are those with the highest penetration of some type of incineration.
Ash from modern incinerators is vitrified at temperatures of 1,000–1,100 °C (1,830–2,010 °F), reducing the leachability and toxicity of residues. As a result, special landfills generally are no longer required for incinerator ash from municipal waste streams. Some regions also recover glass, stone, ceramic materials, and ferrous and nonferrous metals used in construction. The volume of combusted waste is less than that of noncombusted waste, thus extending the lifespan of landfills and reducing the needs for municipalities to construct new landfills or export wastes. Other factors to consider are the many forms of “carbon capture” technology in development. Flue–carbon scrubbers might one day improve greatly the value of that, albeit linear, technology (12).
Recycle
Converted Material Recovery (Figure 1a): One of the most successful postuse mechanical “recycling” approaches for SUTs is to repurpose used plastic into such products as construction lumber, architectural devices, and shipping pallets. Such practice often is termed downcycling, and in a first iteration, it cuts environmental burden per kilogram of virgin plastic in half. The approach is possible without SUT decontamination, but concerns include
• its need for waste to be transported to the few facilities providing the service
• its one-way application, never again feeding the material needs for highly stringent applications
• its limited mass throughput.
Vendors can sell only a limited number of reclaimed plastic boards or pallets. However, developing new ways of using downcycled materials could provide a secondary market that could have both financial and environmental benefits.
Polymer Recycling: An attractive approach that has become prevalent for some materials (e.g., polyethylene terephthalate, and high-density polyethylene) in some settings is the processing of the constituent polymer for reuse in other products. Often termed mechanical or simple chemical recycling, that includes different approaches to the preparation and cleaning of the polymer prescribed to produce a usable plastic resin. Although no approach is completely efficient, filtering and otherwise decontaminating a thermally melted (Figure 1B) or solvent-liquified (Figure 1C) polymer can result in a resin of sufficient quality for many final products. Polymer recycling does have limitations, including
• a need for sorting and selecting the particular pieces that are acceptable for this use
• a potential for degradation of the polymers and monomers in both the first use and cleaning process
• potential difficulty in validating second-use products for all purposes.
Monomer Recycling: An up-and-coming technology is the chemical recycling of plastic monomer. This approach exists with different goals, chemistries, and variations, but they can be regarded as existing in two basic technologies: decomposition into hydrocarbon mixtures (e.g., naphtha) and more specific depolymerization into constituent monomeric compounds. Each approach can be accomplished chemically (Figure 1D) or enzymatically (Figure 1G). In general, the process involves breaking down plastic polymers into polymer-constituent monomers and using those to produce “virgin” polymer resin. Another method gaining practicality for some applications is methanolysis, which is a transesterification to monomers. Methanolysis facilities are being constructed to process polyester and polyethylene terephthalate to monomers (13).
| Examples of Thermolytic Processes
Pyrolysis involves heat and no oxygen. Product spectrum varies with such factors as Carbonization involves high heat and no oxygen and is the extreme outcome of pyrolysis at high temperatures. Solid products predominate. Gasification converts polymers to small molecules with some oxygen present. Full Methanization uses synthesis gas to form methanol. Other syngas reactions include the production of larger hydrocarbons (e.g., Fischer–Tropsch reaction). These are the basic processes, but catalysts and other chemistries yield other products. For example, syngas production involves the production of carbon dioxide. The water gas shift reaction can convert carbon dioxide and hydrogen to carbon monoxide and water. Gasification plants based on coal feedstock typically make use of the water–gas shift reaction and Fischer–Tropsch synthesis to create products. |
Monomers can be chemically produced from polymers through pyrolysis and gasification (Figure 1E, F) and organically by bacteria or enzymes (Figure 1G, H). Thermolytic approaches (see box above) such as pyrolysis and gasification involve the same basic process used to produce fuels, but they can yield different products for applications such as reagent material feedstock for new polymer synthesis or activated carbon. In fact, today most methanol is made from methane through syngas produced from crude petroleum. However, the three processes detailed above do not adequately describe the new reactions and associated derivatives now produced through gasification.
Creative chemistries have been used to produce syngases of disparate composition and even sequester the carbon during syngas formation (14). Such creative approaches can be implemented in gasification toward desired reagent production. Generating chemicals or elements for use in the synthesis of useful products, however, requires the input of a significant amount of energy.
Approaches with engineered enzymes (Figure 1G) and organic or bacterial systems of cloned procaryotic strains (Figure 1H) to decompose used plastic have been described and even validated in small scales. However, these technologies require significant investment to develop the processes at commercial scales (15, 16).
Elemental Recycling: The above recycling methods can result in the significant loss of materials, such as those from UV-induced decomposition of resin components or from oxidation, which produces nonproductive by-products. Thus, recycling the elemental constituents of plastic is the only way to establish a truly circular economy of plastic. For example, plastics broken down at temperatures >1,000 °C in an environment of limited oxygen (Figure 1F) produces syngas of mostly molecular hydrogen, carbon dioxide, carbon monoxide, and methane. That syngas can be used to produce new compounds such as plastic resins. In that way, plastics can be recycled virtually indefinitely, eliminating the need to use terrestrially sourced petroleum. Three significant limitations to that approach are the energy consumed in transport of used plastic to a chemical recycling center, the enormous energy required to break down the plastic, and the storage of breakdown products for use in anabolic or synthetic reactions to new materials. The levels of industrial maturity also can differ by geographic regions.
Need for Further Studies
Over this three-part series, we have elucidated impactful conclusions regarding the relative environmental burden presented by using SUTs in biomanufacturing. Our most significant findings are the following:
• SUTs provide unique health and safety benefits and generally less environmentally impactful processes.
• Science-based examinations of SUT design, manufacture, shipping, and use can yield further improvements.
• Postuse handling of plastics is a small part of providing a sustainable solution to the use of SUT (1, 4).
Herein, we presented an overview of current and near-future postuse destinations of plastic materials. Options for more sustainable operations are being developed. However, for reasons such as shipping distances, volume of material generated, local capabilities, and regional laws, current practical solutions to SUT postuse handling are best evaluated case by case. Even as more technologies become commercially available at scale, satisfactory solutions for a particular waste stream might remain elusive. A comprehensive study is needed to compare both the existing environmental burdens modulated in a proposed solution with trade-offs generated in light of established corporate sustainability goals and time frames.
In general, establishing new, sustainable SUT value chains will require new business models; updated regulations; and new chemical, physical, and digital technologies. Those solutions will require a sincere effort by all players to work collaboratively toward science-based, efficient, and economical candidate technologies. Successful candidates should be determined by considering long-term ecological, economic, and sociological consequences.
No solutions providing a true circular economy are yet available. Ultimately, they will require an overhaul of the design, use, and processing of our materials streams. However, exciting progress toward more sustainable options is apparent. The BPSA is dedicated to reporting on currently available options and promising research developments in the environmentally friendly design, use, and postuse handing of SUTs.
References
1 Barbaroux M, et al. The Green Imperative: Part One – Life Cycle Assessment and Sustainability for Single-Use Technologies in the Biopharmaceutical Industry. BioProcess Int. 18(6) 2020: 12–18; https://lne.e92.mwp.accessdomain.com/manufacturing/single-use/the-green-imperative-part-one-life-cycle-assessment-postuse-processing-and-sustainability-for-single-use-technologies-in-the-biopharmaceutical-industry.
2 Single-Use Technology and Sustainability: Quantifying the Environmental Impact in Biologic Manufacturing. Cytiva LIfe Sciencies (formerly GE Healthcare Life Sciences): Uppsala, Sweden, 2017; https://cdn.cytivalifesciences.com/dmm3bwsv3/AssetStream.aspx?mediaformatid=10061&destinationid=10016&assetid=16801.
3 Sauve G, Van Acker K. Integrating Life Cycle Assessment (LCA) and Quantitative Risk Assessment (qra) to Address Model Uncertainties: Defining a Landfill Reference Case Under Varying Environmental and Engineering Conditions. Int. J. Life Cycle Assess. 26(3) 2021: 591–603; https://doi.org/10.1007/s11367-020-01848-z.
4 Barbaroux M, et al. Green Imperative: Part Two — Engineering for Sustainability in Single-Use Technologies. BioProcess Int. 19(1–2) 2021: 18–25; https://lne.e92.mwp.accessdomain.com/manufacturing/single-use/the-green-imperative-part-two-engineering-for-the-new-plastics-economy-and-sustainability-in-single-use-technologies.
5 Crippa M, et al. A Circular Economy for Plastics: Insights from Research and Innovation to Inform Policy and Funding Decisions. De Smet M, M. Linder M, Eds. European Commission: Brussels, Belgium, 2019; https://op.europa.eu/en/publication-detail/-/publication/33251cf9-3b0b-11e9-8d04-01aa75ed71a1/language-en/format-PDF/source-87705298.
6 SUT Glossary. Bio-Process Systems Alliance Sustainability Committee; https://bpsalliance.org/wp-content/uploads/2021/10/BPSA-Sustainability-Committee-Glossary.pdf.
7 Themelis NJ. An Overview of the Global Waste-to-Energy Industry. Waste Manage. World (July–August 2003): 40–47; https://waste-management-world.com/a/an-overview-of-the-global-waste-to-energy-industry.
8 ASQ 834 2021 0115: What Is the Most Common Disposition Method for Your Company’s Single-Use Waste? Aspen Alert 3378; 3 February 2021. https://www.aspenmediainc.com/archive/category/surveys?id=30.
9 Royte E. Is Burning Plastic Waste a Good Idea? Nat. Geo. 12 March 2019; https://www.nationalgeographic.com/environment/article/should-we-burn-plastic-waste.
10 Pinto Da Costa J, Rocha-Santos T, Duarte A. The Environmental Impacts of Plastics and Microplastics Use, Waste, and Pollution: EU and National Measures. European Parliament PE 658.279, October 2020; https://www.europarl.europa.eu/RegData/etudes/STUD/2020/658279/IPOL_STU(2020)658279_EN.pdf.
11 Municipal Waste Management Across European Countries. European Environmental Agency: Copenhagen, Denmark, 15 November 2016; https://www.eea.europa.eu/publications/municipal-waste-management-across-european-countries/copy_of_municipal-waste-management-across-european-countries.
12 Adlen E, Hepburn C. 10 Carbon Capture Methods Compared: Costs, Scalability, Permanence, Cleanness. Energy Post, 11 November 2019; https://energypost.eu/10-carbon-capture-methods-compared-costs-scalability-permanence-cleanness.
13 Toto D, Eastman Invests in Methanolysis Plant in Kingsport, Tennessee. Recycling Today, February 1 2021 – https://www.recyclingtoday.com/article/eastman-chemical-recycling-plastics-investment.
14 Jie X, et al. Microwave-Initiated Catalytic Deconstruction of Plastic Waste into Hydrogen and High-Value Carbons. Nat. Catal. 3, 2020: 902–912; https://doi.org/10.1038/s41929-020-00518-5.
15 Tournier V, et al. An Engineered PET Depolymerase to Break Down and Recycle Plastic Bottles. Nature 580, 2020: 216–219; https://doi.org/10.1038/s41586-020-2149-4.
16 Mohanan N, et al. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 26 November 2020; https://doi.org/10.3389/fmicb.2020.580709.
17 Bensona NU, Bassey DE, Palanisamic T. COVID Pollution: Impact of COVID-19 Pandemic on Global Plastic Waste Footprint. Helyon 7(2) 2021: e06343; https://doi.org/10.1016/j.heliyon.2021.e06343.
18 Ignacio J. From Single-Use to Re-Use.The Medicine Maker June 2018; https://themedicinemaker.com/manufacture/from-single-use-to-re-use.
19 Shyns Z, Shaver M. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 42(3) 2021: e2000415; https://doi.org/10.1002/marc.202000415.
20 Zhaoa Y-B, Lv X-D, Nia H-G. Solvent-Based Separation and Recycling of Waste Plastics: A Review. Chemosphere 209, 2018: 707–720; https://doi.org/10.1016/j.chemosphere.2018.06.095.
21 Dissolution: Extracting Plastic. Cefic, The European Chemical Industry Council: Brussels, Belgium, 2021; https://cefic.org/a-solution-provider-for-sustainability/chemical-recycling-making-plastics-circular/chemical-recycling-via-dissolution-to-plastic.
22 Coates GW, Getzler YDYL. Chemical Recycling to Monomer for an Ideal, Circular Polymer Economy. Nat. Rev. Mater. 5, 2020: 501–516; https://doi.org/10.1038/s41578-020-0190-4.
23 Depolymerisation: Breaking It Down To Basic Building Blocks. Cefic, The European Chemical Industry Council: Brussels, Belgium, 2021; https://cefic.org/a-solution-provider-for-sustainability/chemical-recycling-making-plastics-circular/chemical-recycling-via-depolymerisation-to-monomer.
24 Kaminsky W. Recycling of Polymers By Pyrolysis. J. Physique IV Proceed. 03(C7) 1993; C7-1543–C7-1552; https://hal.archives-ouvertes.fr/jpa-00251879/document.
25 Brems, A. et al. Gasification Of Plastic Waste As Waste-To-Energy or Waste-To- Syngas Recovery Route. Nat. Sci. 5(6) 2013: 695–704; https://doi.org/ 10.4236/ns.2013.56086.
26 Biodegradable and Compostable Plastics: Challenges and Opportunities. European Environment Agency: Copenhagen, Denmark, 27 August 2020; https://www.eea.europa.eu/publications/biodegradable-and-compostable-plastics/biodegradable-and-compostable-plastics-challenges.
27 Chamas A, et al. Degradation Rates of Plastics in the Environment. ACS Sustainable Chem. Eng. 8(9) 2020: 3494–3511; https://doi.org/10.1021/acssuschemeng.9b06635.
28 Bisinella V, et al. Environmental Assessment of Carbon Capture and Storage (CCS) as a Posttreatment Technology in Waste Incineration. Waste Manag. 128(3) 2021: 99–113; https://doi.org/10.1016/j.wasman.2021.04.046.
Corresponding author William Whitford is life science strategic solutions leader for DPS Group in Logan, UT; william.whitford@dpsgroupglobal.com. Sade Mokuolu is strategy implementation manager at Watson-Marlow Fluid Technology Group (a Spirax-Sarco Engineering company), Magali Barbaroux is a corporate research fellow at Sartorius, Mitchell Snyder is applications engineering manager at Saint-Gobain Bioprocess Solutions, and Mark Petrich is both vice president of technical operations at Krystal Biotech, Inc. and first vice chair of the Bio-Process Systems Alliance (BPSA).