Endotoxin — also known as lipopolysaccharide (LPS) — is a large molecule made up of a lipid and a polysaccharide portion found in the outer membrane of each Gram-negative bacterial cell. Examination of endotoxins is necessary for microbial and animal cell-culture operations that produce active pharmaceutical ingredients (APIs) and vaccines. If a cell culture is contaminated with endotoxins, it could affect cell growth and function (thus potentially affecting product quality). Biopharmaceutical companies need to understand the endotoxin levels within their…
Manufacturing
eBook: Raw Material Control Strategy — Leveraging Knowledge of Material Attributes and Data Analytics as Key Elements
Ensuring pharmaceutical quality begins with in-depth understanding of process/platform capabilities, which is informed by knowledge gained through product and process development, subject-matter expertise, and lessons learned from experience. And all outside factors that can affect manufacturing outcomes must be taken into consideration. Extra vigilance is necessary for understanding potential sources of variation and maintaining robust control strategies to ensure process consistency — and ultimately product quality for patients. Biomanufacturing unit operations require multiple raw materials that must be documented as…
eBook: Antibody–Drug Conjugates — Refining Product Designs for Improved Outcomes
Antibody–drug conjugates (ADCs) seek to partner the target specificity of antibodies with the cell-killing punch of chemotherapy drugs. Researchers identify antibodies that bind to proteins found predominantly or exclusively on the surfaces of cancer cells. The cells can absorb the ADC into their interiors, where the chemical environment or enzymes detach the drug from the antibody, freeing it to wreak havoc. Although nine ADCs have received US Food and Drug Administration (FDA) or European Medicines Agency (EMA) approval (and many…
eBook: Viral Vector Purification — A Discussion of Current Challenges and Methods
Adenoassociated viral (AAV) vectors have become synonymous with gene therapy delivery. However, because they are produced in such small quantities and because their upstream processes carry comparatively large amounts of host-cell DNA and other impurities, AAV purification can be challenging. Several researchers have applied different chromatographic strategies, but no universal method has been adopted in the biopharmaceutical industry. This eBook features a discussion among several industry experts that explores challenges specific to AAV purification, shedding light on whether current strategies…
eBook: Trends in Facility Design — In-House Manufacturing Considerations for Cell and Gene Therapy Production
Manufacturing and facility challenges facing cell and gene therapy companies are similar to but more complex than those encountered by companies that produce traditional biopharmaceuticals such as vaccines, monoclonal antibodies, and other therapeutic proteins. A single product can have multiple components, manufacturing of which may or may not be outsourced. Project timelines are short, production technologies are new and evolving, and clinical demands change rapidly. Increasing competition for contract manufacturing services requires reserving capacity far in advance, which in most…
New Data Analytics Tools Can Improve Bioprocess Workflows — If Applied Correctly
Biotherapeutics are a hot topic right now — and for good reason. But even before the COVID-19 pandemic, the biotherapeutics field, like every other manufacturing sector, was exploding with all the data that are being generated by recent innovations in equipment, systems, and processes. Advances in biomanufacturing analytics, analytical technology, and machine learning have tried to keep pace; however, such tools too often are misunderstood and applied suboptimally. Thus, many companies struggle with confusion and missed opportunities when they should…
Unraveling the Complexities of Technology Transfer
In the biopharmaceutical industry, technology transfer refers to transfer of any process, together with its documentation and professional expertise, between development and manufacture or between manufacturing sites (1). This operation is common in the biopharmaceutical industry for a number of structural reasons. They include the dichotomy between small, innovation-based drug companies and large ones able to conduct late-phase clinical development and endowed with manufacturing capacity; the high capital cost of biopharmaceutical plants, which makes contract manufacturing attractive; and the need…
Developing Advanced-Therapy Products Through Global CDMOs
Tremendous growth in the cell and gene therapy (CGT) industry is driving unprecedented demand for manufacturing services. To be sure, advanced-therapy developers increasingly are choosing to install in-house capabilities. Doing so can offer companies greater control of their processes, timelines, and budgets than they might have when outsourcing products (1). But industry experts agree that contract development and manufacturing organizations (CDMOs) will remain integral to CGT manufacturing and commercialization (1, 2), especially with veteran contract partners scrambling to acquire CGT…
Dissolved Oxygen Control Tuning for Cell Culture Applications
Proper tuning of dissolved oxygen (DO) controller proportional integral (PI) values is essential for optimal cell culture performance in a bioreactor. When DO-PI values are optimized, gas flows are smoothed, and foaming and cell stress are reduced. Traditionally, this tuning has been performed by using nitrogen gas to purge oxygen from a test solution, thus simulating oxygen demand. That method has several drawbacks, however. First, nitrogen gassing cannot simulate the high demands of high-density fermentation. Second, nitrogen competes with other…
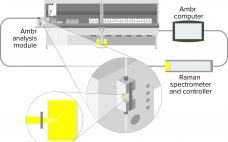
Novel Integrated Raman Spectroscopy Technology for Minibioreactors: Accelerating Raman Model Building for Cell Culture Monitoring and Control
Raman spectroscopy is used widely in biomanufacturing as a process analytical technology (PAT) for monitoring analytes such as glucose and lactate (1). Predictive Raman models also can be used to control glucose concentration in cell cultures (2). The technique is becoming more popular for pilot- and manufacturing-scale bioreactors, but it only recently has been studied with minibioreactors for measuring analytes and producing predictive Raman models for feedback control (3) thanks to advances in integrated technology for automating sampling, analysis, and…