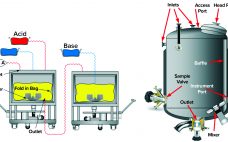
Read about the challenges of sterile filtration of high concentration mAbs, liposomes, and lentiviral vectors, and how to solve them in this Special Report. Development of new, complex drug formulations has given us therapeutics with properties that are markedly different from traditional drug types. High viscosity or low surface tension formulations or large viral vector molecules can mean that sterile filtration processes, which are optimized for traditional drug types, are not as efficient for the new, complex formulations. Premature filter…
Manufacturing
Drug Formulations Are Changing:
Making MAbs: Bioprocess Advancements Challenge Platform Assumptions
Still the largest sector of the industry, monoclonal antibodies (MAbs) have dominated the biopharmaceutical stage for over 30 years. Some observers might think there’s nothing new to say about these molecules; others point to antibody derivatives as a more exciting alternative. But MAbs are far from an outdated technology. From biosimilar developments to cell-free synthesis to yeast display, immunogenicity improvements, and fully human antibodies — as well as improvements in process efficiency and cost reductions, as discussed herein — the…
Tracking Therapeutic Antibody Development in a Pandemic
The COVID-19 pandemic has generated a significant and rapid response from scientists who aim to develop drugs and vaccines in the academic, government, and industrial sectors. Such interventions are essential to containing SARS-CoV-2, the coronavirus that causes the COVID-19 disease. To inform and educate the public about global efforts to develop targeted therapies such as monoclonal antibodies (MAbs), The Antibody Society (TAS) and the Chinese Antibody Society (CAS) have designed and implemented a freely available online database called the COVID-19…
G-Protein–Coupled Receptors: Promising Targets for Antibody–Drug Conjugates
G-protein–coupled receptors (GPCRs) are a large and diverse family of seven-transmembrane–domain proteins expressed on the surface of human cells. These molecules respond to external stimuli by initiating signal-transduction pathways that affect the expression of a large family of genes — which, in turn, regulate a range of vital physiological processes and functions. Figure 1 illustrates the general pathways of GPCRs. Without these proteins, humans simply could not survive: Without β-adrenergic receptors, we could not regulate our blood sugar, for example;…
The Crossroads of Academia, ​Industry, and Education: Modern Training Centers Are Pivotal to the Future of R&D
Global pharmaceutical industry research and development (R&D) investment has experienced steady growth over the past two decades, with an anticipated compound annual growth rate (CAGR) of 3.0% and projected 2024 investment of US$213 billion (1). Focused on developing innovative therapies for chronic, infectious, genetic, and lifestyle-related ailments, the fast-growing biologics segment has become a cornerstone of the pharmaceutical industry and healthcare sector. The demonstrated effectiveness and wide-ranging applicability of biopharmaceuticals also have brought considerable R&D in computational and biological technologies.…
Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies, Part 1: Upstream and Downstream Strategies
Future biomanufacturing must address industry drivers, including the need for decreasing cost of goods (CoG), increasing market globalization, shortening development time for pipeline products, reducing risk to patient supply, and improving product quality. A CASSS chemistry, manufacturing, and controls (CMC) forum entitled “Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies” took place on 16–17 July 2018 in Gaithersburg, MD, to address those opportunities. Advanced technologies include single-use bioreactors, alternating tangential-flow (ATF) systems used during fermentation, modular and closed process equipment,…
A New Runway for Purification of Messenger RNA
A high-performing capture method is a critical bedrock asset for developing industrial purification processes. This is especially true for extended families of products that share highly similar chemical composition. Therapeutic monoclonal IgG is an example. The ability of protein A affinity chromatography to achieve 95% purity in one simple step was the runway that got recombinant immunotherapy off the ground and made it available to millions. In fact, protein A did more. Beyond giving the industry a foundation manufacturing method,…
How Much Harm Can a Single Droplet Do? Considerations for a Viral Inactivation Step
Viral clearance is a fundamental aspect of viral safety for biopharmaceutical products. Regulatory agencies around the world require biomanufacturers to segregate their operations appropriately to mitigate the risks of carryover contamination from previous process steps or product batches and of crossover contamination between product(s) made in the same facility. Guidelines are vague in defining “appropriate,” leaving biomanufacturers to interpret regulatory expectations and define their own virus reduction and segregation strategies. Given the differences among manufacturing processes and facilities housing such…
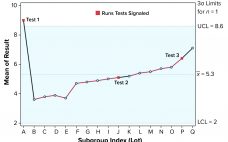
Run Rules with Autocorrelated Data for Continued Process Verification
Control charts can be used to assist in monitoring of biopharmaceutical product quality attributes as part of continued process verification activities. A number of tests known as run rules have been developed to assess whether biomanufacturing processes remain in statistical control. In practice, results for such attributes can be positively autocorrelated. Simulated data are used to assess the performance of run rules with autocorrelated data to assist in determining risk–reward profiles for process monitoring. Autocorrelated Data The tendency for data…
Technology to Transform AAV Manufacture
Adenoassociated virus (AAV) vectors are a popular choice for modern gene therapies because of their favorable safety profile, low immunogenicity, and the ease with which they can be transduced into different cell and tissue types. An AAV genome is a single strand of DNA comprising a replication (rep) gene, which encodes regulatory proteins involved in genome replication, and a capsid (cap) gene, which produces three capsid proteins. However, AAVs cannot replicate alone. In nature, AAV shares an exquisite relationship with…