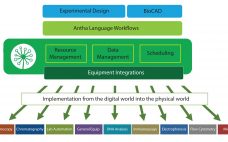
Synthace began as a bioprocess optimization company in 2011, spun out of University College, London. The company worked on multifactorial approaches with 15–30 factors simultaneously instead of seven or eight. The work investigated genetic strain engineering factors alongside process parameters, defining deep interactions between the way strains were designed and the way they were treated in bioprocesses. Those complex experiments gave unique insight into the complexities of biological processes, but they were exceptionally taxing to plan and carryout manually. Automation…
Information Technology
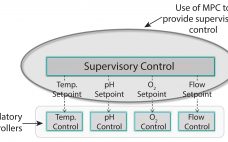
Model Predictive Control for Bioprocess Forecasting and Optimization
Automation hierarchy in bioprocess manufacturing consists of a regulatory layer, process analytics technology (PAT), and (potentially) a top-level model-predictive or supervisory layer. The regulatory layer is responsible for keeping typical process measurements such as temperature, pressure, flows, and pH on target. In some cases, spectral instrumentation in combination with multivariate analysis (MVA) can be configured to measure parameters such as glucose concentration. A cascade control structure can be set up when the nutrient flow setpoint is adjusted to maintain the…
Data Analysis and Visualization to Improve Biopharmaceutical Operations Part 1: What Are You Trying To Measure?
This begins a five-article series of “how-to” guides for tackling the most common obstacles in assessing, measuring, analyzing, and improving the performance of global biopharmaceutical manufacturing operations. Each installment covers a component of proper collection, analysis, and use of data for the best possible performance outcomes. When taken as a whole, the series should provide imperative best practices for handling business-performance data. First, consider what you want to know about your bioprocesses. How can you more appropriately measure those data…
The Era of Digital Biomanufacturing
The digital revolution in manufacturing began with an explosion in monitoring, analytics, and new computing capabilities. Combined with such advances as artificial intelligence (AI), automation, and robotics, they are changing our concepts of manufacturing in general — from product development and factory operations to materials supply. This evolution also connects product and process designers and leaders in manufacturing engineering. Digital manufacturing (DM) isn’t a dream or a concept on some advanced developer’s design table; it’s occurring now and will change…
Harnessing the Power of Big Data to Improve Drug R&D
Like many other industries, biotechnology is being transformed by the emergence of big data — extremely large data sets that can be analyzed to reveal patterns, trends, and associations — and advanced analytics. Information from multiple sources such as electronic health records, payer claims, and mobile health platforms is growing exponentially. When used and harnessed properly, these data can boost the efficiency of drug research and development (R&D) in three critical areas: early R&D investment, drug development, and personalized medicine.…
Flexible Automation for Continuous Unit Operations
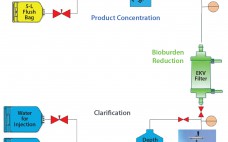
Continuous processing has the potential to provide significant cost and time savings for biopharmaceutical manufacturing, but that potential can be realized only if appropriate automation solutions are available for continuous flow between disparate upstream and downstream operations. Pall Life Sciences’ Allegro MVP system, a fully automated bioprocessing system designed for use in upstream and downstream single-use processing, enables flexible automation and thus facilitates continuous biopharmaceutical manufacturing. This article presents the results obtained using the Allegro MVP system in combination with…
The Year of Data Integrity: 2015 Brought a Worldwide Focus on Training, System Design and Control, and Data Management
Each year, regulatory agencies from around the world focus on critical aspects of the pharmaceutical quality management system, bringing awareness to the industry and continuing to effect positive change. In the past five years, risk assessments, electronic records, and outsourced activities have been in the spotlight. As 2015 closed out, it was clearly the year of data integrity. In March 2015, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) published its GMP Data Integrity Definitions and Guidance for Industry,…
Elucidation: Strict, but Flexible, Industrial Automation for Biopharmaceutical Manufacturing
Biopharmaceuticals are the fastest growing sector of the pharmaceutical industry, making up about 20% of the market, with annual growth rates of about 8% (double that of more traditional pharmaceutical sectors). To increase capacity and uphold stringent quality and regulatory demands, manufacturers often reassess their operational and technology strategies while focusing on rising manufacturing costs and the pressure of delivering cost-effective new drug products. Bioproduction can range from small batches to low-cost, high-volume campaigns. Few manufacturers have the required in-house…
From Chips to CHO Cells: IT Advances in Upstream Bioprocessing
Advances in our capabilities for data acquisition, storage, and manipulation are providing the biopharmaceutical industry with an increased understanding of what must be controlled in bioproduction as well as the ability to control it. Developments in hardware, processing algorithms, and software are changing the landscape of bioprocess administration. Increased power for information gathering and processing began with the remarkable increases in microprocessor speed, pipelining, and parallelism over the past couple of decades (1); it continues with advances in data handling…
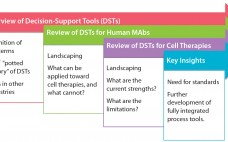
Decision-Support Tools for Monoclonal Antibody and Cell Therapy Bioprocessing: Current Landscape and Development Opportunities
Industrial-scale manufacturers in a number of fields — from automobiles to biotherapeutics — have long relied on powerful computational and mathematical tools to aid in the scale-up, optimization, quality control, and monitoring of product development (1–5). Typical process pathways are highly multifactorial, with numerous branch points, feedback steps, instrumental attributes, and target parameters. Moreover, margins for error are minimal for most industrial processes, requiring high standards of precision from industrial and operational pathways (6). For those reasons, the complexity of…