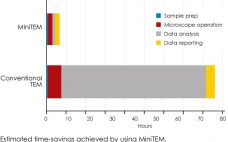
MiniTEM⢠is a low-voltage transmission electron microscope system designed for nanoparticle characterization. The high-quality images it acquires reveal particle morphologies that can be transformed into accurate metrics. A metric for sample purity based on the ratio of the total area of debris to that of intact Adenovirus particles is presented in this study. The automated analysis that MiniTEM offers encompasses a far larger number of particles compared with studies performed manually using conventional electron microscopy, resulting in accurate and statistically…
Downstream
An Example of Evaluation of protein A Multicolumn Processes for the Capture of Large-Volume, High Concentration Bioreactors, Based on BioSC® Predict Optimization Software
The present paper provides some recent data regarding the optimization of BioSC® processes using three commercially available protein A resins, based on the BioSC® Predict software. A rapid description of the first steps to develop a multi-column process guided by simulation is provided to avoid generating unrealistic conclusions. Other approaches could however be applied depending on the objectives targeted. Examples of multicolumn systems based on three commercially available protein A resins are also provided, for the purification of a 15,000-L…
Fast monoclonal antibody titer determination with the TSKgel® Protein A-5PW column
The antibody therapeutics market is enjoying high growth rates, the major areas of therapeutic application being cancer and immune/inflammation-related disorders. Six of the top ten best-selling global drug brands are monoclonal antibody-based. This market is predicated to show continued growth for many years to come, with more monoclonal antibodies (mAbs) designed and produced for treatments of specific diseases. Early in mAb development there are many harvested cell supernatant samples which contain mAbs that are secreted by cell cultures. These samples…
TOYOPEARL NH2-750F: Flow-through Removal of Endotoxin
Endotoxins are remnants of bacterial cell walls that may contaminate drug products and cause an immunogenic response. They are often referred to as âpyrogensâ due to their fever-inducing effects. Endotoxins may be found in drug products either due to contamination from host cells used to produce a drug product in a bacterial expression system or due to adventitious bacterial contamination in non-microbial products. Thus, endotoxin clearance is a requirement of downstream processing of biologics, especially those derived from microbial expression…
A comparative study of MiniTEM⢠versus DLS Zetasizer Nano and NTA NanoSight NS300 performance when analyzing < 50 nm particles
Characterization and analysis of subvisible particles of biological origin can be challenging or give insufficient information. In this study Adeno associated virus (AAV) particles contaminated by host cell proteasomes are analysed with direct versus indirect methods performed in a standard laboratory setting to reveal the difference in performance and quality of the information obtained. The focus is on the ability to detect the AAV particles of interest and distinguish them from the contaminating proteasomes. The compact, tabletop MiniTEM system enables…
Understanding Single-Pass Tangential Flow Filtration and the New Era of Bioprocessing
Learn how single-pass tangential flow filtration (SPTFF) technology is revolutionizing current and future bioprocessing platforms with its implementations in biotech, vaccine and plasma industries. Adopting SPTFF at process development (PD), pilot, clinical and commercial manufacturing scales has major advantages over conventional approaches. Key benefits include flexible manufacturing, reduced processing volumes, high product recoveries and increased yield, as well as major savings in capital expenditures. SPTFF also enables disposable and/or single-use technology utilization.
Pall SoloHill® Animal Protein-Free Microcarriers for Dengue Virus Production
Pall Life Sciences offers a variety of regulatory-friendly animal protein-free (APF) SoloHill® microcarriers that can be quickly and easily optimized for use in vaccine production platforms. Our application note describes dengue virus production in Vero, optimizing cell culture seeding densities and microcarrier working concentrations for efficient cell attachment and growth in two different cell culture media platforms: a traditional serum supplemented formulation and a commercially available serum-free formulation (SFM). Overall, the results of this study demonstrates that Pall APF microcarriers…
Taking Advantage of High Capacity Protein A: An Economic Comparison of the Resin Cost Per Gram of Antibody Produced
With pressures mounting to reduce production costs at many companies, and protein A being the most expensive resin used in mAb purification, the use of a high capacity protein A resin can significantly impact the overall cost of doing business. This report details how using a high capacity protein A resin will reduce production costs, on a per-gram produced basis, for companies that implement its use in their chromatography platform.
Robust, Load Independent Viral Clearance in Monoclonal Antibody Purification
Anion exchange chromatography is an important flow-through polishing step for viral clearance of monoclonal antibodies. Resin based materials are most commonly used for this but the large size of virus particles limits diffusion and binding capacity. It is therefore necessary to use large volumes of resin and oversized column hardware. Column chromatography as a unit operation also means high operational costs, including validation, cleaning and storage. This is a particular issue for clinical manufacturing, where a column is used for…
Increased Monoclonal Antibody Resolution with TSKgel® UP-SW3000 Columns
The antibody therapeutics market is enjoying high growth rates, the major areas of therapeutic application being cancer and immune/inflammation-related disorders including arthritis and multiple sclerosis. In 2013, six of the top ten best-selling global drug brands were monoclonal antibodies (mAbs) and more than 400 mAbs were in clinical trials. The characterization of these complex biomolecules is a major challenge in process monitoring and quality control. The main product characteristics to be monitored are aggregate and fragment content, glycosylation pattern and…