Happy 2022 to all of BPI’s loyal and supportive authors, readers, and advertisers. Despite continuing pandemic impediments, your industry continues to show remarkable flexibility and resilience. As technologies evolved to meet the demands for vaccines, tests, and treatments for COVID-19, new hybrid options have opened up opportunities for people to meet and collaborate. Given the changing fortunes of our own publishing industry, managing this hybrid, controlled-publication model (peer-review + trade/B2B content) requires some agility of our own. So it should…
January-February 2022
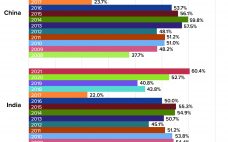
Bioprocessing Facilities in Asia Consider Domestic Alternatives to Western Suppliers
The global biopharmaceutical industry had been growing robustly even before the COVID-19 pandemic. According to the BioPlan Associates Top 1000 Biofacility Index and Biomanufacturers Database (1), bioprocessing capacity worldwide increased an average of 12% over the past decade. China has seen nearly double that rate. India’s bioprocessing segment also is showing strong growth. Because those regions represent 37% of the world’s population — and with rapidly growing middle-class economies, — demand for biologics there is outstripping that elsewhere. Historically, Western…
In Search of a CMO for My Biotechnology Startup: How to Navigate a Journey Without Process Maps
An innovative biopharmaceutical product can transform from an abstract idea at small scale into the basis of a burgeoning startup company. At that point, company leaders seek ways to ensure that a biologic will scale up in a quality-controlled, professional, and sustainable environment. That involves refining a research-stage prototype into a product that will be consistent and reproducible for research and development (R&D) and manufacturing and that will meet all relevant regulatory standards in specified target markets. Within the constraints…
Posttranslational Modifications and Their Control in CHO Culture
The Chinese hamster ovary (CHO) cell line was first established by Theodore Puck in the 1950s and was used mainly for cytogenetics studies (1). Since the 1990s, CHO cells have increased in popularity as expression host cells because they can be adapted easily into suspension culture and genetically modified. The CHO cell line also has a human-like glycosylation profile (2–4). Therapeutic proteins undergo different posttranslational modifications (PTMs) during manufacturing. Some modifications can lead to undesired effects such as decreased stability,…
Analyzing Single-Use Polymers for Cell Culture Processes: Comparison of Cell Growth and Viability Test Procedures
The acceptance and implementation of single-use systems (SUS) or “disposables” has increased strongly in bioprocess development and biopharmaceutical manufacturing over the past two decades. Typically, suppliers provide SUS presterilized and ready to use. Using SUS eliminates time-consuming and expensive cleaning procedures (which often require corrosive chemicals and a large amounts of water) and removes the need to perform cleaning validation between batches. The application of SUS reduces the risk of product cross-contamination and increases product and patient safety (1–5). Polypropylene…
Establishing a Digital Platform for Data Science Applications in Biopharmaceutical Manufacturing
Biopharmaceutical manufacturing consists of multiple processes with complex unit operations. Those include mammalian cell culture in upstream operations and downstream chromatography steps for removing impurities from production streams and purifying the therapeutic biological molecule (1). Biomanufacturers need enhanced understanding to ensure the process control and manufacture of safe and efficacious drug products. Process understanding also enables opportunities for improving manufacturing efficiency. Both process understanding and optimization can be facilitated by leveraging large volumes of biotechnology data — typically generated during…
Ask the Expert: A Scalable Platform for Production of High-Quality and GMP-Grade Plasmid DNA
Demand continues to grow for clinical- and good manufacturing practice (GMP)-grade plasmid DNA (pDNA) amid the proliferation of therapies based on mRNA and viral vectors. At a newly acquired manufacturing facility in San Diego, CA, Wacker Chemie is expanding its capabilities with a scalable platform for pDNA production. In October 2021, Mack Kuo (associate director of bioprocess development at Wacker Biotech US) showcased the site’s capabilities and described how it can provide comprehensive, customizable services for plasmid production. Kuo’s Presentation…
Ask the Expert: Bolstering Manufacturing Capacity and Achieving Supply-Chain Resilience
The novel coronavirus pandemic has exposed vulnerabilities in biopharmaceutical industry approaches to supply-chain management. Drug developers and manufacturers have tended to presume suppliers’ access to raw materials for bioprocess components. However, global crises disrupt reliable access. Ari Ojinaka (production manager at Astrea Bioseparations) joined BPI in October 2021 to explore strategies for navigating supply-chain uncertainty. He described how his company seeks to optimize manufacturing capacity, enhance communication with its suppliers and customers, and support a quality-driven company culture. Ojinaka’s Presentation…
Ask the Expert: Scale-Up Success for Cell Cultures in Single-Use Benchtop Bioreactors
During process development (PD), single-use (SU) bioreactors can streamline cell-culture workflows and reduce risks for contamination and operator errors. But transferring cultures from laboratory- to benchtop- and pilot-scale vessels requires consideration of fundamental bioreactor principles. In October 2021, Ann D’Ambruoso (manager of product applications and marketing) and Cristina Bernal Martinez (applications support engineer, both at Getinge) reviewed factors for scale-up success and presented a case study involving transfer of cell cultures to Applikon AppliFlex SU stirred-tank (ST) reactors. The Presentations…
Ask the Expert: Optimized Cell Line Development for CHO DG44 Expression Systems
In November 2021, Rathangadhara Nammalwar (manager of protein-based therapeutics at Sartorius) delivered a presentation describing the hallmarks of a robust cell line development (CLD) platform. Focusing on the importance of expression constructs, he then explained how collaboration with external partners has enabled Sartorius to optimize its Cellca CLD platform for mammalian-cell production of therapeutic proteins. Nammalwar’s Presentation Nammalwar identified key criteria for selecting a CLD platform, noting first that it should leverage a highly productive cell line that yields high-quality…