With autumn beginning to assert itself, our days here in Oregon are cooler, and long-awaited rain is coming. From drought and wildfires to tropical storms and flooding around the globe, we hope that you and your loved ones are making it through this year’s extreme weather events unscathed. And we continue to wish you well through the continuing healthcare crisis. Autumn is time for the annual BPI Conference (including its parallel cell and gene therapy track), held as a hybrid…
October 2021
Collaboration Agreements: Critical Issues and Common Pitfalls
The number of collaboration arrangements for emerging technologies is increasing significantly, especially among companies developing COVID-19 diagnostics, vaccines, and therapeutics. Consequently, a massive amount of capital was invested in the biopharmaceutical industry in 2020. These deals range in value from the low millions to several billion dollars, representing significant risks and value for investors. Some recipients of that capital are seeking partners with whom to collaborate, sharing both the costs and risks associated with their activities and bringing innovative technologies…
Implementation of Established Conditions: Learnings from a “Sharing Science Solutions” Workshop
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline Q12 (1) (step 4 sign-off in November 2019) is in the process of being implemented in a number of regulatory regions. The document provides additional frameworks for pharmaceutical life-cycle management. It is intended to support globally harmonized regulatory tools such as established conditions (ECs) and product life cycle management (PLCM) documents to facilitate postapproval changes to chemistry, manufacturing, and controls (CMC). Although a harmonized framework…
Intellectual Property and COVID-19
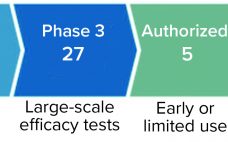
The COVID-19 pandemic is the greatest global health threat caused by a virus since the influenza pandemic of 1918 (and, before that, innumerable smallpox outbreaks throughout history). So far, the number of infections and deaths has not reached levels seen during the “Spanish flu” pandemic. However, travel, global trade, and modern factors such as misinformation on social media have increased infection rates and risks of infection. As of 15 July 2021, the World Health Organization (WHO) had reported 188,655,968 confirmed…
Designing Vaccines: The Role of Artificial Intelligence and Digital Health, Part 1
According to the founder and executive chairman of the World Economic Forum, Klaus Schwab, the fourth industrial revolution began in the 21st century and is characterized by an unprecedented development and exponential growth of a high-technology industry transforming society at every level (1–4). In particular, healthcare systems are evolving rapidly to adapt to the new reality. According to Forbes, the main technologies currently shifting the paradigm of medical research are artificial intelligence (AI) and machine learning (ML) (5), both defined…
MAb Viral Clearance Studies:
A Substantiated Platform Approach for the IND Stage
Virus removal/inactivation is a major concern in the safety of monoclonal antibodies (MAbs) and other recombinant-protein drugs. Some methods (such as nanofiltration and low-pH inactivation) have been demonstrated repeatedly by the industry to be reliable for most viruses, with >4 log10 removal. Based on my company’s virus-removal experiences with its MAb downstream-process platform, we propose a “bracketing method” — testing only samples that lie at the extremes of a design space — to prove proactively that small differences in operating…
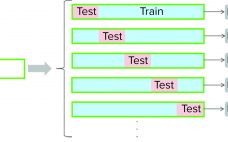
Multivariate Data-Driven Modeling for Continued Process Verification
Continued process verification (CPV) is an integral part of process validation for the manufacture of human and animal drugs and biological products (1). It is designed to meet three primary goals: maintain a validated state of products, their processes, and related systems; enable continuous process improvements; and meet regulatory requirements for life-cycle validation. A CPV program for a biologic product entails regular collection of data related to critical process parameters (CPPs) and critical quality attributes (CQAs) and the preprocessing, analysis,…
Risk Determination of Potential Mycotoxin Exposure to Patients: Testing Recombinant Human Factor VII from Transgenic Rabbits
Sevenfact eptacog beta is a new recombinant human factor VIIa (rFVIIa) developed by LFB SA in Les Ulis, France, as a bypassing agent (BPA) for treatment and control of bleeding in people with hemophilia A and B and inhibitors (1, 2). The product was approved for use in adults and adolescents by the US Food and Drug Administration (FDA) in April 2020 (3). It is expressed in the milk of transgenic rabbits and purified through a multistep process using both…
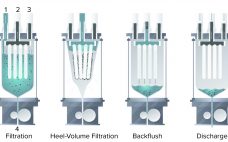
Comparing Single-Use Multicycle Cake Filtration with Depth Filtration: Eliminating the Downstream Bottleneck
Over the past few decades, single-use (SU) technology has increased bioproduction efficiency significantly, especially with the introduction of disposable bioreactors in upstream processing. To keep pace with major developments and increases in upstream capacity, downstream processes also must increase capacity and efficiency. However, cell harvesting and downstream processing continue to present bottlenecks in manufacturing (1). Typical clarification processes are composed of primary and secondary clarification steps, such as centrifugation followed by depth filtration, respectively (1). Two sets of SU depth…
Transfection Best Practices for AAV Gene Therapy Programs
As viral vectors continue to push gene therapy innovations closer to market, many researchers are setting their sights on optimizing transfection, the process of delivering corrective genetic material into cells. It’s not just a question of how to transfect them, but also how to do so efficiently and at high volumes. Approaches that work for one cell line might not perform well for others, and transfection protocols can have different implications for scalability and cost during production for clinical trials.…