Short time frames are a major challenge in developing alternative microbiological methods for autologous cell therapy products. Ideally, results are made available in under a day. Obtaining regulatory acceptance also can be a challenge, but it is made easier if methods are included in an application (e.g., a biologics license application, BLA) rather than changing a method that is already part of an approved process. Comparing different detection platforms can be a challenge if they have different readouts, and validation…
2017
Comparing Adherent-Cell Technologies: Amplification of Virus Stocks and Viral Vectors
FUJIFILM Diosynth Biotechnology (FDB) is a multifunctional commercial development manufacturing organization (CDMO) that manufactures virus-based therapeutics. Its site in Texas specializes in both adherent and suspension cell culture for production of virus vectors and for development of analytical assays to support production and expression of our clients’ therapeutics. We work with a number of cell lines such as human embryonic kidney (HEK293) and human retinal cells (Johnson & Johnson/Crucell’s proprietary PER.C6 line), which can grow in suspension. However, many of…
December From the Editors
On the night of Wednesday, 27 September 2017, the fourth annual Battle of the Biotech Bands raised US$110,000 for the bands’ selected charities. More than 800 guests enjoyed refreshments at the Royale Nightclub in Boston and watched Led Zymmelin (pictured right, representing Sanofi Genzyme) walk away with the top prize. They raised money for the National Organization for Rare Disorders (NORD). The crowd was a mix of Biotech Week Boston attendees; arts and entertainment critics; developers, contractors, architects, engineers, furniture…
December Spotlight
Revealed: Where Scientists Want to Work When you’re in the business of life-science innovation, highly trained employees are worth every penny. That’s why biotechnology companies pay top dollar for amenities-rich locations and facilities in the top US life-science real estate markets, according to Jones Lang LaSalle’s (JLL’s) Life Sciences Outlook published this past summer. JLL is a professional services firm that specializes in real estate and investment management. Its report says that life-science professionals have high expectations for their workplaces.…
Serialization: Background, Justification, Requirements, Timelines, and Readiness Across the Supply Chain
Drug manufacturers are facing unprecedented serialization challenges. Serialization requires weighty consideration and focused strategy for successful commercialization, even for those companies that have yet to bring a product to market. The World Health Organization estimates that 10% of medicines worldwide and up to 50% of drugs consumed in developing nations are counterfeit. In response to increasing drug integrity concerns, more than 40 countries have introduced laws mandating serialization and tracing of pharmaceutical products as they pass through the supply chain.…
Implementation of the BPOG Extractables Testing Protocols: Working with Multiple Single-Use Components
Single-use technologies offer significant advantages over traditional stainless-steel solutions for biopharmaceutical manufacturing. Reductions in setup times, cleaning and cleaning-validation costs, elimination of cross-contamination risks, and smaller footprints are just some of the benefits they provide. Although adoption of single-use systems (SUS) for commercial manufacturing is expanding, concerns persist that extractable and leachable (E&L) compounds from plastic SUS components potentially can leach into final drug products and compromise efficacy and safety. Those concerns are magnified amid the growing number of SUS…
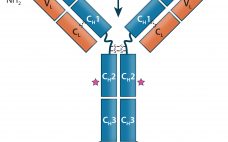
Therapeutic IgG-Like Bispecific Antibodies: Modular Versatility and Manufacturing Challenges, Part 1
Antibody-based immunotherapy has advanced significantly since 1986, when the US Food and Drug Administration (FDA) approved the first mouse monoclonal antibody (MAb) for clinical use: Orthoclone OKT-3 (muromonab-CD3). In the intervening years, researchers have applied the tools of genetic engineering to clone immunoglobulin G (IgG) genes into a number of expression vectors. In the 1990s, the bioprocess industry was able to produce fully human antibodies in cultured cells. As of June 2017, the FDA and the European Medicines Agency (EMA)…
Streamlined Column-Packing Design for a New Commercial Launch Facility
To meet network demand for a commercial launch facility, Genentech (Roche) designed a new downstream train and built it within an existing building shell at the company’s Oceanside, CA, site. This downstream train included new technologies to allow for rapid technology transfer of different new products in the company’s drug pipeline. One technology that was pursued was the Axichrom column platform from GE Healthcare and associated column packing equipment to streamline column packing design. Here we focus on how a…
Addressing the Challenge of Complex Buffer Management: An In-Line Conditioning Collaboration
Read this article from scientists at Cytiva (GE Healthcare at the time of publication) to learn more about buffer preparation strategies now. Preparation and storage of buffers is a challenge for biopharmaceutical companies developing protein-based pharmaceuticals. The need for volumes of buffer to purify increasing upstream titers have become a major bottleneck in biopharmaceutical downstream processing. Italian biopharmaceutical company Kedrion Biopharma collects and fractionates blood plasma to produce plasma-derived therapeutic products for treating and preventing serious diseases, disorders, and conditions…
Simplify Upstream Process Development and Scale-Up: Single-Use 5:1 Turndown-Ratio Bioreactor Technology
Single-use technologies (SUTs) have been adopted widely in the biopharmaceutical industry for product development as well as clinical- and commercial-scale manufacturing. Over the years, suppliers of such equipment have addressed concerns about waste management, extractables and leachables, and reliability of supply — and as a result, end users have gained confidence in SUTs. Recognizing potential benefits that can be realized for both clinical and commercial operations, biomanufacturers increasingly are implementing SU solutions at larger scales in both upstream production and…