Getting biologic drugs through development and into clinical proof-of-concept studies quickly and efficiently is critical for success in the biopharmaceutical industry. Implementing high-throughput approaches to both upstream and downstream process development is increasingly helping companies stay competitive. Innovative and highthroughput analytical technologies are needed to support rapid process development. The study reported herein focuses on innovative immunoassay platforms for impurity-removal monitoring of both host-cell proteins (HCPs) and leached protein A.
HCPs come from host cells during cell culture production. Their compositions and amounts in final drug products depend highly upon multiple factors, including the expression system, culture conditions, and purification process. Removal to safe levels during downstream purification is imperative to prevent adverse clinical effects. Immunogenicity, a critical adverse effect, may be directly correlated with the amount of certain HCPs in the product, considering that even small amounts of HCPs can be highly immunogenic (1, 2). Consequently, regulatory authorities require close monitoring of HCP concentrations in intermediate and final biologic products. Typically, they suggest reducing levels to parts per million (<100 ppm) (3). That often depends on the product dose and route of administration as well as the production system.
Leached protein A is a process-related impurity introduced during manufacturing processes that include protein A affinity chromatography. Protein A can leach from affinity resins and coelute with immunoglobulin (IgG) antibodies as a ProA–IgG complex (4). Because protein A is both immunogenic (5) and mitogenic (6), regulators require its clearance to acceptable levels, typically below the detection limit of a given assay, during biologic purification processes.
Traditional enzyme-linked immunosorbent assays (ELISA) are the most common method for quantification of impurities. The technique is conventionally accepted by regulatory agencies and currently considered to be the biopharmaceutical industry’s “gold standard” for quantifying HCPs and leached protein A in antibody production. Polyclonal antibodies are raised against complex preparations of protein A and HCPs by immunizing an antibody-producing species (e.g., goat, sheep, rabbit, or chicken) (7). The resulting array of antibodies form the basis of this sandwich-format immune-enzymatic assay. The method is often labor-intensive, however, with a narrow dynamic range and limited sample throughput.
Alternative ELISA-based technologies have been developed to address the industry’s need for improved throughput and increased dynamic ranges. Among these approaches are the amplified luminescent proximity homogeneous assay (ALPHA), homologous time-resolved fluorescence (HTRF), mesoscale discovery (MSD), electrochemiluminescence technology (ECL), and acoustic membrane microparticle (AMMP) technologies (8, 9). All are suitable for detection of HCPs and in some cases for leached protein A. But sample throughput and/or turnaround are still limiting factors.
Herein, we summarize our evaluation of three emerging high-throughput technologies for quantitation of leached protein A and HCPs: ForteBio’s Octet platform, Gyrolab workstations from Gyros, and Thermo Fisher Scientific’s solid-phase proximity-ligation assay (SP-PLA) (Figure 1) (10–12). We not only evaluated sample throughput and turn-around times, but also performed a comprehensive assessment of hands-on time, cost, and overall assay performance regarding range, sensitivity, and precision. We tested multiple monoclonal antibodies (MAbs) from different in-process steps using both ELISAs and the new approaches. Implementation of new technologies often must negotiate the hurdle of new equipment expense, development, and training cost, as well as instrument maintenance. So one key consideration for our laboratory is implementation of platform technologies for many different products in development. Thus we investigated the applicability of each option for both HCP and protein A impurity monitoring.
Materials and Equipment
We tested multiple in-process and bulk drug substance antibody products from Boehringer Ingelheim for comparability using all four methods.
ELISA: The HCP ELISA kit came from Cygnus Technologies (catalog #F550), and all HCP kits provided with the different technologies (Octet, SP-PLA, Gyrolab) are based on the same polyclonal antibodies from that company. Our analysis followed instructions provided with the HCP ELISA kit using an HCP standard provided in the kit. We purchased protein A ELISA capture and detection antibodies from Cygnus Technologies, as well (catalog #I060 and #F401, respectively). For acid dissociation of the ProA–IgG complex, we used phosphate-buffered saline (PBS) with 0.02% Tween 20, adjusted to pH 3.5 with acetic acid. The calibration standard we used in leached protein A determination was rProtein A-Cys ligand under restricted license (catalog #28-4018-59) from GE Healthcare. Both horseradish peroxidase (HRP) conjugated anti-HCP/anti–protein-A detection antibodies use enzyme substrate 3, 3′, 5, 5′-tetramethylbenzidine (TMB) to produce a visible signal for detection, read by a standard Spectramax plate reader from Molecular Devices LLC.
SP-PL Assay: For the SP-PLA method, we purchased ProteinSEQ HCP and ProteinSEQ protein A (catalog #4469343) kits (catalog #A27601 and #4469343, respectively) from Thermo Fisher Scientific. The kit came with an HCP standard, and we purchased a protein A (MabSelect) standard from GE Healthcare (catalog #28401859). We used a Thermo Fisher MagMAX system to process the HCP and protein A capture, wash, antibody binding, wash, and ligation steps. For the real time qPCR analysis, we used a Thermo Fisher 7500 fast real-time PCR system. Sample testing followed instructions provided in the ProteinSEQ kits. For the leached ProA assay, we diluted samples in the dissociation buffer provided and boiled them at 100 °C for 10 minutes, then cooled them down to room temperature (five minutes) and centrifuged them for five minutes at 16,000g to pellet precipitated IgG. Supernatants were further processed with the MagMAX and qPCR instruments according to the SP-PLA kit instructions.
Octet System: HCP and protein A kits purchased from ForteBio included Streptavidin biosensors for the Octet assays (catalog #18-5075, Dip and Read residual protein A kit; #18-5081, Dip and Read anti-CHO HCP detection kit). We used an Octet RED96 workstation, which applies the Dip and Read biosensors with specific capture antibodies immobilized on their tip surfaces for detection and quantification of specified proteins. We ran the HCP assay according to the kit protocol. But to dissociate the ProA–IgG complex, we used acid dissociation instead of the boiling method recommended in the kit protocol (see ELISA method above).
Gyrolab Technology: We purchased buffers and leached protein A antibodies from Gyros and goat anti-HCP antibodies from Cygnus Technologies. To label the capture antibodies, we used a biotinylation reagent (EZ-Link Sulfo-NHS-LC-Biotin) from Pierce Biotechnology and a reactive dye (Alexa 647) from Thermal Fisher Scientific to label the detection antibodies. A Gyrolab xP workstation automated these immunoassays using microfluidic compact discs (CDs) with fluorescence detection. We used Bioaffy 200 and Gyrolab Mixing CDs for HCP and protein A, respectively.
Methods and Results
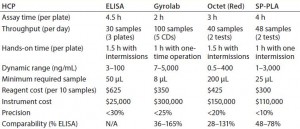
Table 1: Comparing assay capacity, parameters, cost, and performance of investigated technologies for the HCP assay
ELISA: For the traditional HCP ELISA, we developed a Cygnus 3G HCP ELISA kit as a partially homogeneous assay. Capture and detection antibodies are added at the same time as samples for incubation. Remaining reagents are washed out before TMB is added. This method is still the gold standard in our laboratory, but it has low throughput with only 2 logs of dynamic range (3–100 ng/mL) (Table 1). Samples with varying HCP concentrations (≤1,000,000 ppm in cell culture fluid, 1 ppm in final drug substance) must be diluted to a desired range. Because certain HCP species are underestimated because of capture antibody saturation (the “hook effect”), additional dilutions are often warranted. Those multiple serial dilutions (required to ensure that enough data points fall into the dynamic range of the standard curve) are a main reason for the low sample throughput of labor-intensive ELISAs. Assay precision is <30%.
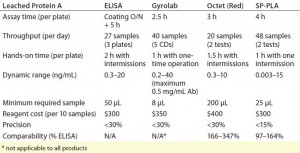
Table 2: Comparing assay capacity, parameters, cost, and performance of investigated technologies for the protein A assay; for instrument cost, see Table 1.
Sandwich ELISAs also are typically used to monitor removal of leached protein A during process development and in final drug products. One concern regarding this assay is that MAbs present in samples tend to form complexes with leached protein A, often interfering with analysis. The Cygnus kit suggests a boiling method for dissociation of those complexes to expose the protein A molecules for efficient binding to capture and detection antibodies. But we have seen poor recovery with that procedure. So we performed an acid-dissociation step to separate protein A from MAb products, which provided acceptable protein A recovery yields (>70%, except for one product’s 45%). Due to the same considerations as stated for the HCP ELISA above, the ELISA for leached protein A is a low-throughput assay with a narrow dynamic range of 0.3–20 ng/mL (Table 2). This assay’s precision is <30% as well.
Octet Method: ForteBio’s Octet system uses bilayer interferometry (BLI) technology, a proprietary optical analytical technology, to monitor biomolecular interactions in real-time (13). The instrument analyzes the interference pattern of white light reflected from two surfaces: a layer of immobilized protein on the biosensor tip, and an internal reference layer. Any change in the number of molecules bound to the biosensor tip (e.g., during construction of a sandwich format) causes a shift in the interference pattern that can be measured. This technology is an established methodology for binding-type analysis, with an impurity-assay application having been developed recently (13).
ForteBio offers assay kits for detection of HCPs and protein A. As with the ELISA kit from Cygnus, the company recommends a boiling procedure for dissociation of ProA– IgG complexes as part of the assay protocol for leached protein A. Again, we found that boiling procedure to be unsuccessful for all samples tested, with poor protein A recovery for certain antibody products. So we used the same acidic buffer here as in our protein A ELISA protocol to dissociate the ProA–IgG complexes.
We observed no considerable improvement in the hands-on time spent with this method compared with that of a traditional ELISA (Table 1, Table 2). For both approaches, sample preparation must be performed up front, including dilutions and/or dissociation of the ProA–IgG complexes. Overall assay time is decreased with the OctetRED96 instrument by its automated sample incubation and readout. Limiting factors for this method include reagent cost (highest among all technologies tested) and a large required sample volume of 200 µL.
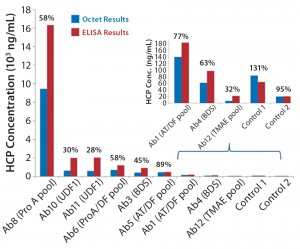
Figure 2: Comparing absolute HCP values (ng/mL) obtained using Octet system with results measured by ELISA for different process steps throughout purification; percentages shown are Octet results relative to ELISA.
Our investigation demonstrated good dilutional linearity for HCP detection, as well as good assay precision (20%, compared with 30% in ELISA) and a 1-log increase in dynamic range (0.5–400 ng/mL compared with 3–100 ng/mL for ELISA). Sample throughput thus was increased (40 samples in two tests) through a reduction in required sample-dilution points. Octet HCP results were 28–131% compared with ELISA throughout its process steps (Figure 2). Because the polyclonal antibodies used in all these technologies come from the same source, all differences observed are based on technological differences alone. We postulate that the observed differences here are partly attributable to different binding behaviors of those antibodies on the much smaller biosensor tip surface area than in an ELISA plate well’s surface area.
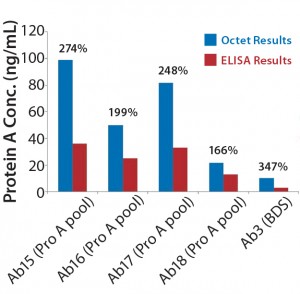
Figure 3: Comparing absolute leached protein A values (ng/mL) obtained using Octet system with results measured by ELISA for different process steps throughout purification; percentages shown are Octet results relative to ELISA.
For the protein A kit, the Octet performance is similar to that of an ELISA in terms of throughput, linear range, and precision — except for the benefit of a reduced assay time (Table 2). Octet results varied by 166–347% compared with ELISA results (Figure 3).
Gyrolab Technology: The Gyrolab xP workstation is a unique automated analytical platform based on microfluidic technology (8, 14). Immunoassays are automatically controlled in identical microstructures within a Gyrolab CD, in which an affinity-capture column containing streptavidin-coated beads is used to capture biotinylated antibodies. All samples and reagents are transferred to each microstructure by spinning the CD at precisely controlled speeds. Samples are processed simultaneously in parallel, and quantification is achieved through integration of laser-induced fluorescence detection.
The binding of target molecules on 15-nL affinity-capture columns in flow-through mode reduces incubation times and minimizes matrix effects (15). That shortens assay times and reduces required dilution points per sample. Assay time was cut in half compared with a traditional ELISA, with increased sample throughput and reduced hands-on time (Table 1, Table 2). Because of their nanoliter scale, Gyrolab assays require only small amounts of samples and reagents (several microliters), which considerably lowers sample consumption compared with the traditional assay. Consumption of the relatively expensive reagents also is reduced, but depending on throughput, the overall cost can be comparable to the ELISA assay.
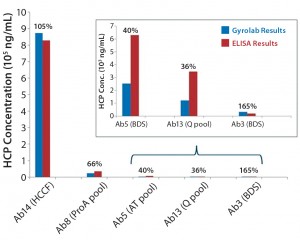
Figure 4: Comparing absolute HCP values (ng/mL) obtained using Gyrolab system with results measured by ELISA for different process steps throughout purification; percentages shown are Gyrolab results relative to ELISA.
For the HCP assay, we observed an impressive dynamic range of >4 logs. The volumes delivered to the microstructures were well controlled by the spinning speed of the CD, which resulted in improved precision (<25%) compared with the ELISA format (<30%). When comparing the absolute values to the gold standard ELISA assay, the results ranged from 36% to 165% (Figure 4). The HCP immunoassay format is based on polyclonal-antibody interactions with a complex mixture of many HCP species in varying amounts, so results may differ between the dynamic flow-through environment of a Gyrolab assay and the more static environment of antibody reactions in plate-based ELISAs.
Testing for leached protein A on a Gyrolab workstation similarly demonstrates faster assay time and lowers hands-on requirements than the ELISA. However, we found that this method was not applicable to all of our evaluated antibody products. For those that showed promising results, samples needed to be diluted to a maximum concentration of 0.5 mg/mL antibody, which ultimately affected the limit of quantitation (LoQ) for highly concentrated samples. In collaboration with Gyros, we tried a number of approaches but were unable to resolve the problem for certain MAbs in our portfolio. It is notable that the polyclonal protein A antibodies used for this assay are the vendor’s own proprietary antibodies.
SP-PL Assay: SP-PLA uses proximity-ligation technology to convert antibody–target-molecule binding into reporter nucleic acid molecules, with detection of ligation products by real-time PCR (16, 17). Target molecules captured on functionalized magnetic beads are recognized by two specific antibodies that are conjugated with oligonucleotide proximity probes. The probe ends are brought into proximity as antibody components bind to target molecules, then allowed to hybridize to a short connector oligonucleotide so that oligonucleotides on the probes can be joined through enzymatic ligation. Then ligation products are amplified and detected with quantitative PCR (qPCR). Unlike the single antibody-binding event detected by ELISA, SP-PLA requires detection antibodies to bind concurrently to two different epitopes of the same target molecule. That requirement for recognition by three antibodies should reduce nonspecific/crossreactive antibody binding and therefore allow for high specificity and sensitivity.
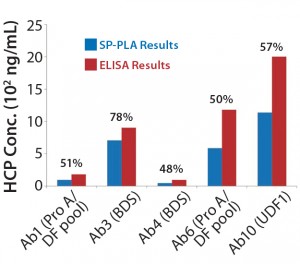
Figure 5: Comparing absolute HCP values (ng/mL) obtained using SP-PLA with results measured by ELISA for different process steps throughout purification; percentages shown are SP-PLA results relative to ELISA.
We found the dynamic range to be significantly increased for both HCP (1–3,000 ng/mL) and protein A (0.003–15 ng/mL) SP-PLAs compared with ELISAs (HCP 3–100 ng/mL; protein A 0.3–20 ng/mL) (Table 1, Table 2). For the HCP assay, this resulted in a threefold-reduced LoQ with a significantly widened upper range. Sensitivity of the leached protein A assay increased 100-fold, with an upper range comparable to that of the ELISA assay. We observed no significant matrix interference. The proprietary boiling dissociation of ProA–IgG complexes contains a carrier and was very effective.
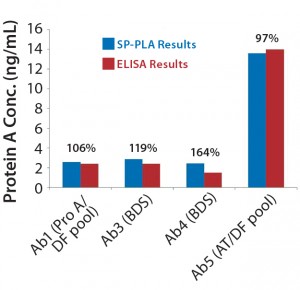
Figure 6: Comparing absolute leached protein A values (ng/mL) obtained using SP-PLA with results measured by ELISA for the different process steps throughout purification; percentages shown are SP-PLA results relative to ELISA.
All products we tested with this method showed >70% spike recovery yield, whereas the ELISA spike recovery was 45% for one product tested (data not shown). The high sensitivity of the SP-PLA technique allows for dilution of samples to pg/mL levels of leached protein A, which further eliminates matrix or IgG interference. Assay precision for both HCP and protein A formats was within 10–15%, which is considerably better than that for ELISA. Absolute results compare closer to ELISA results than any of the other techniques, with 48–78% and 97–164% for HCP and leached protein A, respectively (Figure 5, Figure 6). Overall sample throughput increases about twofold because the large dynamic range (4 logs) and good dilutional linearity decreases the sample-dilution requirement.
Discussion
Our study showed that all evaluated technologies are suitable for monitoring leached protein A and HCP impurities. The performance of each is either significantly improved or comparable to the “gold-standard” ELISA assay, depending on evaluated parameters such as cost, assay time, hands-on time, sample throughput, linear range, or precision. Each technology has its pros and cons, depending on the purpose and priorities of different laboratories (e.g., high throughput, precision, technology platforms, and so on).
We found the Gyrolab workstation to be a useful tool for high-throughput screening with its significantly reduced assay time and impressive sample throughput. For the HCP assay, those parameters were unmet by any other technology, and it showed a good precision of <25% and a 4-log dynamic range. Similar advantages also have been reported by companies such as Bristol-Myers Squibb, Pfizer, Merck, Lonza, and Medimmune (8, 9, 14, 18–20). We found a minor disadvantage with it to be a slightly higher LoQ of 7 ng/mL compared with 3 ng/mL for the ELISA. Furthermore, Gyrolab results show variation of 36–165% compared with ELISA results. Although we can make no statement about the absolute accuracy of either method, that difference in measurements could lead to poor correlation with a final process if ELISAs are used for release. Nevertheless, the software is 21 CFR Part 11 compliant. For new projects, this technology could be implemented in a good manufacturing/laboratory practice (GMP/GLP) environment, which would alleviate that concern.
Limited information has been published about the Gyolab assay for leached protein A, and most of what’s available has been provided by Gyros and GE themselves (21). Boehringer Ingelheim was among the first beta testers, using the protein A kit with mixing CDs for acid-dissociation automation. For our investigated products, unfortunately, we observed varying levels of matrix interference. IgG tolerance proved to be <0.5 mg/mL to achieve acceptable linearity for two of our antibodies, whereas our evaluation of others was unsuccessful. So for our products, at this point we are unable to implement the Gyros technology as a platform for impurity monitoring. The company is continuing to improve its assay kits, allowing for a possibility of future evaluation and implementation. We also have demonstrated the suitability of the Gyros xP workstation for titer measurements (22) as an alternative application.
Finally, the Gyrolab instrument costs US$300,000, which is double or triple the cost of the other technologies. That up-front cost eventually could be off-set by reduced reagent cost, depending on estimated sample throughputs. Also, Gyros recently introduced a smaller Gyrolab xPlore instrument at a lower cost, although with reduced throughput (one CD rather than five CDs).
Although its throughput or dynamic range were not as impressively improved as the other new technologies, the Octet system provides a robust alternative to traditional ELISA methods. It offers additional improvements in precision and sample time, as have been reported by others for the HCP application (9). Furthermore, ForteBio has since released Octet RED384 and Octet HTX systems, both with significantly improved throughput over that of the Octet RED96 system we used. We found a considerable advantage of the Octet instrument in that it demonstrated suitability as a platform technology for impurity monitoring of both HCP and leached protein A. In addition, it is also a standard instrumentation used in GMP/GLP environments for analysis of binding and kinetics studies (13). Limiting factors include its slightly higher reagent costs and large minimum sample volume of 200 µL (for the Octet RED96 system). The latter is especially of concern with small-scale, high-throughput process development studies with limited sample availability. Finally, as described for the Gyros system above, the discrepancy between Octet and ELISA results could lead to poor correlation with a final process.
Application of SP-PLA to detection and quantification of HCPs and leached protein A is still fairly new (16, 17). Only the HCP kit is available from Thermo Scientific now; the ProA kit will be released soon. The SP-PLA method does not drastically improve throughput (twofold increase over ELISA), but we demonstrated excellent assay performance with a broad (4 log) dynamic range for both assays, while additionally showing outstanding sensitivity for the protein A assay with 0.003 ng/mL. The latter is two logs lower than for the other techniques we tested. We found a considerable improvement in assay precision of 10–15% as well as overall good comparison with ELISA results. Depending on the assay format (HCP or protein A), the overall hands-on time is only slightly improved. The actual benefit in assay time depends on operator preference and workflow. Additional assay time could be reduced by combining capture and ligation steps, thus shortening the script of the MagMAX sample-preparation procedure. Like with the Octet method, this technology not only is suitable as a platform for both immunoassay types, but the associated equipment also can be shared further within the GxP world’s well-established residual DNA analysis by qPCR.
Although the technologies we investigated do offer significant advantages over the traditional ELISA method, none emerges as an overall solution. Depending on the needs of your laboratory, we advise weighing the importance of sample throughput, workflow, platform requirements, cost, assay performance, and comparability with ELISA results. These technologies are constantly evolving, so advancements in the near future might tip the balance to one or another.
References
1 Chamberlain P. Immunogenicity of Therapeutic Proteins. The Reg. Rev. 5 (5–6), 2002.
2 Eaton LC. Host-Cell Contaminant Protein Assay Development for Recombinant Biopharmaceuticals. J. Chromatogr. A 705(1) 1995: 105–114.
3 Hogwood CEM, et al. Measurement and Control of Host-Cell Proteins (HCPs) in CHO Cell Bioprocesses. Curr. Opin. Biotechnol. 30(c) 2014: 153–160.
4 Zhu-Shimoni J. Trace Level Analysis of Leached Protein A in Bioprocess Samples without Interference from the Large Excess of rhMAb IgG. J. Immunol. Meth. 341(1–2) 2009: 59–67.
5 Gomez MI, Lee A. Staphylococcus aureus protein A Induces Airway Epithelial Inflammatory Responses By Activating TNFR1. Nat. Med. 10(8) 2004: 842–848.
6 Bensinger WI, et al. Clinical Trials with Staphylococcal Protein A. J. Biol. Response Modif. 3(3) 1984: 347.
7 Jin M, et al. Profiling of Host-Cell Proteins By Two-Dimensional Difference Gel Electrophoresis (2D-DIGE): Implications for Downstream Process Development. Biotechnol. Bioeng. 105(2) 2010: 306–316.
8 Mora J et al. Application of the Gyrolab Platform to Ligand-Binding Assays: A User’s Perspective. Bioanalysis 2(10) 2010: 1711–1715.
9 Jackson M, et al. Comparative HCP Testing Using Traditional and Emerging Technologies (poster). Gyros AB: Uppsala, Sweden, 2009; www.gyros.com/why-gyros/knowledge-center/downloads.
10 Furuya K, et al. How Analytics Are Keeping Pace with Process Development: Innovative Technologies to Achieve Rapid and Robust Process Development and Integrating Process and Analytical Knowledge. BioProcess International Conference and Exhibition: Boston, MA, 2013.
11 Zhang L, et al. High-Throughput Methods Evaluation for Impurities Determination during In-Process Development (poster). BioProcess International Conference and Exhibition: Boston, MA, November 2014.
12 Zhang L, et al. Evaluation of Rapid Quantitation Methods for Titer, HCP, and ProA By Gyrolab Platform During In-Process Development. BioProcess International Conference and Exhibition: Boston, MA, November 2014, October 2015.
13 BLI Technology. Pall ForteBio LLC: Menlo Park, CA, 2015; www.fortebio.com/blitechnology.html.
14 Heo JH et al. A Microfluidic Approach to High-Throughput Quantification of Host-Cell Protein Impurities for Bioprocess Development. Pharmaceut. Bioprocess. 2(2) 2014: 129–139.
15 Automated Immunoassays at NanoliterScale. Gyros AB, Uppsala, Sweden, 2015; www.gyros.com/why-gyros/technology.
16 Nan L, et al. Host Cellular Protein Quantification Using an Automated Solid-Phase Proximity Ligation Assay. BioProcess Int. 10(2) 2012: 44–50.
17 Nong R, et al. Solid-Phase Proximity Ligation Assays for Individual or Parallel Protein Analyses with Readout via Real-Time PCR or Sequencing. Nature Protocols 8(6) 2013: 1234–1248.
18 Peiris KJ, et al. Development of Residual Host-Cell Protein Assays for Recombinant Microbial Biopharmaceuticals. 5th Annual European Gyrolab Seminar: Siena, Italy, 2011.
19 Ramsubramaniam N. Rapid Process Development Enabled By Automated, Single Use High-Throughput Technologies. Annual Meeting of the Society for Industrial Microbiology and Biotechnology: Philadelphia, PA, 2015.
20 Roussis M. Automated Quantification of Residual protein A Ligands with Gyrolab Protein A Kit (poster). BioProcess International Conference and Exhibition: Boston, MA, October 2015.
21 Lehtonen P, Inganas M. Quantification of MabSelect SuRe Ligand in Presence of Excess Amounts of IgG on Gyrolab. Gyros AB: Uppsala, Sweden, 2009; www.gyros.com/why-gyros/knowledge-center/references/#impurity.
22 Wang L, et al. Product Quantification (IgG Titer) Using an Automated Analytical Platform — Gyrolab Workstation. Gyros AB: Uppsala, Sweden, 2014; www.gyros.com/why-gyros/knowledge-center/downloads.
Eike Zimmermann, PhD, is an associate director; Lin Wang, PhD, is a scientist II; Anoushka Durve is manager of analytical science; and Kenji Furuya, PhD, is department head of analytical science at Boehringer Ingelheim, 6701 Kaiser Dr, Fremont, CA 94555; 1-510-284-6252; eike. zimmermann@boehringer-ingelheim.com. Formerly a member of the BI team, Fang Li is now a QC specialist at Bayer HealthCare Pharmaceutical, Inc. Formerly a senior associate scientist with the BI team, Li Zhang is now associated director at LakePharma.