This is a transcript from a Q&A interview with Emily Shacter, PhD, Consultant, ThinkFDA LLC (former FDA Scientist and Regulator). We will be talking today about the CMC Forum that was published back in 2005. We are revisiting it in the magazine to specifically update our understanding of how to maintain process control; understanding your process. In general, how do you feel the discussions in the four-part paper from 2005 has held up after 10 years? Emily: I think they…
February 2016
From the Editor: BioProcess International House Style
One of my ongoing projects is to revise our author guidelines for better presentation online. Most publications have formal house-style guidelines to ensure clear and accurate communication. Editors are not immune from making mistakes, but as a reader, I distrust the accuracy of information in text that is (overall) sloppily edited. It is like not wanting to eat in a restaurant with a dirty kitchen. How can I trust the quality of the food? But styles can become outmoded, and…
Spotlight
Niche Disease: Soft-Tissue Sarcoma (STS) by Cheryl Scott Cancer comes in two forms: the more common carcinoma form (of epithelial origin) and the less common sarcoma form. Sarcoma cells are mesenchymal in origin. Soft-tissue sarcomas are cancers of tissues such as fat, muscle, nerves, tendons, the lining of joints, blood vessels, or lymph vessels. Because there are at least 50 different types of STS, it is more accurately described as a family of related diseases rather than a single disease.…
Responding to an FDA Form 483: A Five-Step Approach
When the US Food and Drug Administration (FDA) inspects your company’s biomanufacturing facility, investigators use the FDA Form 483 to record observations and findings (1). Such inspections typically review all good manufacturing practices and good laboratory practices (GxP) quality systems documents. If those investigators find compliance issues, they deliver a summary of their observations and findings using a Form 483, a copy of which will be provided to your company at the end of the inspection visit. How to Respond…
Bridging Analytical Methods for Release and Stability Testing: Technical, Quality and Regulatory Considerations
To monitor the control and consistency of products derived from biological systems, a broad array of analytical methods are used for biopharmaceutical release and stability testing. These methods include both classical and state-of-the-art technologies as well as new technologies as they emerge over time.During the life cycle of a product, several reasons can arise for making changes in existing analytical methods: e.g., improved sensitivity, specificity, or accuracy; increased operational robustness; streamlined workflows; shortened testing times; and lowered cost of testing.…
Process- and Product-Relate Impurities: Part 1 – Process-Related Impurities An Overview
Introduction by Cheryl Scott The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of forum meetings are published in BioProcess International and on the CASSS website (www.casss.org). This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated…
High-Throughput Methods Evaluation: Impurities Determination During Upstream and Downstream In-Process Development
Getting biologic drugs through development and into clinical proof-of-concept studies quickly and efficiently is critical for success in the biopharmaceutical industry. Implementing high-throughput approaches to both upstream and downstream process development is increasingly helping companies stay competitive. Innovative and highthroughput analytical technologies are needed to support rapid process development. The study reported herein focuses on innovative immunoassay platforms for impurity-removal monitoring of both host-cell proteins (HCPs) and leached protein A. HCPs come from host cells during cell culture production. Their…
Using Glycosidases to Remove, Trim, or Modify Glycans on Therapeutic Proteins
One of the most common posttranslational modifications of eukaryotic proteins is glycosylation. Glycosylation of proteins can affect many biological activities. For therapeutic glycoproteins, it can modify biological activity, targeting, trafficking, serum half life, clearance, and recognition by receptors (1, 2). For such reasons, biomanufacturers must monitor and characterize the glycosylation patterns of their recombinant therapeutic proteins (3, 4). Therapeutic proteins have two main types of glycosylation: N-linked glycans and O-linked glycans (5). Attachment of an N-glycan starts in the endoplasmic…
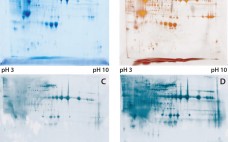
Development of a Novel Host-Cell Protein Assay: Supporting the Physcomitrella patens Expression System
Host-cell proteins (HCPs) constitute an inevitable impurity of biopharmaceutical products originating from recombinant-cell culture. HCPs are a heterogeneous mix of different proteins, their specific characteristics depending on the kind of organism used as an expression platform, on the “destination” of the expressed recombinant product (extra- or intracellular), and on the corresponding purification approach (1–3). Contamination of a final drug substance with residual HCPs could lead to immunogenic reactions in some patients who receive the drug product (DP). So a reliable…
Automated Purification of Native and Recombinant Proteins Using Multidimensional Chromatography
In traditional sequential chromatography, columns are run as separate entities. The process requires significant hands-on time and constant manual intervention. By contrast, automated chromatography technology provides the same results more efficiently and reliably and frees researchers to focus on other tasks, thereby shortening protein purification times from days to hours. For drug discovery, purifying protein samples is required to generate enough materials for research experiments. But the process is complex and time consuming. It involves repeated single-column purifications, careful analysis,…