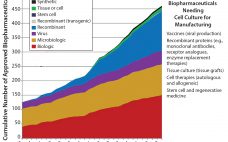
Over the years, the BioProcess International special supplements have tackled a large number of important topics on emerging trends and new technologies in biomanufacturing, including single-use technologies, continuous manufacturing, cell therapies, and outsourcing. As these and other issues continue to arise and play a critical role in the development of the biopharmaceutical industry, the success of the biomanufacturing industry relies on its ability to attract and retain a well-trained and motivated workforce that can adapt to new technologies and manufacturing…
2016
Advancing the Biopharmaceutical Community Through Learning and Development Partnerships
As in most high-tech sectors, success within the highly regulated biopharmaceutical manufacturing industry depends upon an adaptable, skilled, educated, and high-performing workforce. The impact of human performance cuts across the entire industry — whether for a manufacturing operator, a scientist or engineer in a highly technical role such as process development, or a technical sales representative serving the industry. The effects of poor performance can be seen easily in deviation reports and FDA warning letters as well as in quantified…
Designing In-House Training Programs: Moving Beyond Read and Understand
To begin the discussions in this issue about structuring successful training programs, these authors describe how to develop in-house resources. Both emphasize the need to assess the impact of previous training approaches. An important factor is to understand that students will bring varying levels of knowledge and relevant experience to their work with your company. So how do you structure a training program that takes into account individual learning styles and operational expertise? How to you measure the successful application…
Training for New and Emerging Technologies: Building Comprehensive, Process-Oriented Competencies
Assessing the need for training that addresses the varied needs of your employees requires looking far beyond a one-size-fits-all approach. Few companies, however, have the internal resources to develop truly comprehensive programs on their own. Successful training approaches are those that enable end-users, organizations, and suppliers to share needed equipment and expertise. At the 2016 BioProcess Theater at Interphex, Gary Gilleskie (BTEC’s director of operations) and Scott Sommer (a technical fellow at Renmatix) detailed examples of successful training programs for…
Measuring the Impact of Investments in Professional Development: A Virtual Roundtable
Well-designed education, training, and professional development programs and partnerships add considerable return on investment to stakeholders across the biopharmaceutical manufacturing industry. As biomanufacturers, suppliers, and regulatory bodies share similar workforce needs, focused education and training efforts can lead to increases in productivity, yield, and employee engagement and retention. In addition, education and training programs can decrease the incidence of deviations, which are costly to investigate and remediate. Yet when compared with other business needs, training, development, and human performance initiatives…
Exploring Academic Models for Biomanufacturing Education
Undergraduate and/or graduate programs in physical and life sciences can provide a solid background for science and engineering students who are interested in careers in biotechnology research and development. Yet many such programs have not adequately prepared students for careers within and related to the biopharmaceutical industry. In today’s globally competitive job market, developing a workforce pipeline for the bioprocess industry requires academic programs that equip students with knowledge, skills, and theory surrounding the equipment, methodologies, processes, and regulatory requirements…
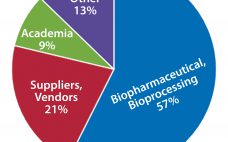
Demographics and Trends: Results from a Joint BPI/BTEC Survey on Training
Training is an investment. And it’s one that the authors, contributors, and other individuals behind this supplement understand well. However, the biopharmaceutical industry is much larger than these few individuals. It’s just as important to understand the motivators, drivers, and training-related challenges faced by the industry at large. So BPI and BTEC jointly administered a short survey to gather some basic information from the magazine’s readership about the value and role that training plays in their organizations. According to survey…
December From the Editor
It is no big revelation that publishing is changing. How people prefer to read and access information is in some ways a demographic conundrum and in other ways an expected technology-driven evolution. Recognizing those changing attitudes and in honor of our 15th anniversary year as a publication, for 2017 BPI is planning to both adapt to and further drive business and publishing trends in ways that truly meet the information needs of our readers around the world. We simply didn’t…
December Spotlight
BPI Advisor Will Be Missed Thomas J. Pritchett, PhD (1950– 2016), was a member of the BioProcess International advisor board since its founding. He also was founder, publisher, and editor of the BioQuality Newsletter and provided decades of analytical and quality control training to many people in the biopharmaceutical industry, both on the industrial and regulatory sides. Pritchett always provided insightful, well–thoughtout approaches to implementing current good manufacturing practices (CGMPs) under all conditions, ranging from virtual to global companies. He…
BioPhorum Operations Group Technology Roadmap, Part 1: Four Trends Shaping the Future of the Industry
What prompts over 100 biopharmaceutical manufacturers, leading academics, supply partner R&D heads, regulators, and worldwide regional hubs to get involved in a major project? It’s when that project identifies the future technology needs of the biopharmaceutical manufacturing industry and accelerates its collective innovation. In February 2015, the BioPhorum Operations Group (BPOG) Technology Roadmapping steering committee met in Washington DC to create the first “technology roadmap” for the biopharmaceutical manufacturing industry. The biopharmaceutical market has been experiencing dramatic changes: explosive growth…