Figure 1: DiscoveRx’s proprietary PathHunter enzyme fragment complementation (EFC) technology consists of the β-galactosidase (β-gal) enzyme split into two inactive components, the enzyme donor peptide (ED) and an enzyme acceptor (EA). When brought together in close proximity, ED complements with EA to form active β-gal. The active enzyme then catalyzes the substrate to generate chemiluminescent light, providing a highly amplified signal to make for a high-sensitivity assay.
With the drug industry’s expanding emphasis on biologics, the need for robust cell-based assays has grown at all stages of development. Requirements for efficacy, quality, and potency testing often demand a complex set of bioassays and/or cell-based assays for new therapeutics or biosimilars. Developers of the latter have found this need for cell-based assays to be particularly challenging.
Commercially available, ready-to-use cell-based assays provide a robust functional response from specific therapeutic targets. They can significantly shorten assay development time while facilitating adoption and greater reproducibility across multiple global sites. Examples discussed herein include cell-based assays for bevacizumab (Avastin), insulin, glucagon-like peptide 1 (GLP1), growth hormone (GH), and anti–tumor-necrosis-factor alpha (anti-TNFα) antibodies. These case studies highlight how commercial tools can reduce assay development time, enabling a biologic developer to move quickly with the goal of getting to market or submission before its competitors.
Bioassays for Potency and Lot-Release Testing
Biologics are complex protein structures that are usually made by and purified from live cells. Such manufacturing processes are highly complicated and can be difficult to control. Lack of control can lead to physicochemical variations that affect drug function. Additionally, biologics are often extremely sensitive to physical conditions (temperature, shear forces, chemical phase, and light) and enzymatic action. These products typically require complex bioassays for batch release and stability assessment rather than (or in addition to) simple chemical tests for identity and purity. So a good measure of biological activity is essential before the drug substance can be released.
Responding to that need, manufacturers often implement a panel of tests for drug-substance and drug-product release. Such assays vary in output, complexity, and time requirements, depending on the specific drug, and they can range from enzymatic tests (e.g., enzyme-linked immunosorbent assays, ELISAs) to cellbased assays (CBAs) and even animal testing. To this end, the industry needs fast, robust, and reproducible cell-based assays that can expedite the process of lot release and stability testing.
Why Cell-Based Assays?
CBAs that test for drug potency offer a number of advantages over animal models or highly complicated in-vitro assays. CBAs typically reduce testing costs by 80–90% compared with testing in animals. Manufacturers can initiate and complete CBAs very quickly, particularly with the advent of ready-to-assay cryopreserved cells, which can cut down on cell culture and hands-on time. The use of clonal cell lines and validated cell banks also dramatically reduces assay variation compared with animal models. (Editor’s Note: Animal testing also faces increasing ethical questions.) For all those and many more reasons, CBAs are becoming the method of choice for potency assay development. However, a question often emerges concerning which CBA or bioassay should be used to test for potency of a given drug.
An ideal potency bioassay should mimic a given therapeutic molecule’s mechanism of action (MoA) while producing highly precise, accurate, and reproducible data in a quality-controlled environment compliant with good laboratory practice (GLP) and/or good manufacturing practice (GMP). Traditionally, bioassay developers have adapted existing phenotypic readouts for their drug targets, often reflecting cellular events that occur far downstream of the target: e.g., cell death, reporter gene expression, cell viability, cytokine release, and cell proliferation. Most such assays require significant retooling or extensive development before they can be implemented for batch release/validation testing, which adds significant time to method development and optimization. In addition, “home-brewed” phenotypic assays have a number of distinct disadvantages ranging from low specificity, long assay time (up to seven days in some cases), high complexity, and variability.
Although immortalized cell lines can be used for potency assays, continuous culturing requires thawing and culturing of a new vial of cells before testing each batch of drugs, which is not ideal in routine quality control (QC) testing. Additionally, cultured cells can change their performance over time through cellular drift, which is unacceptable for a potency or lot-release assay.
Commercially available cell-based potency assays offer a number of advantages over the above-mentioned systems, including reduced development and validation times, easily adopted methods, simplified global transfer processes, reduced overall costs, and global support from their providers. To address the need for such products, companies such as DiscoveRx have developed a broad array of CBAs that have been implemented around the world for potency and lot-release testing.
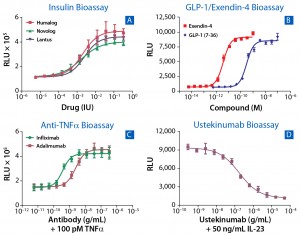
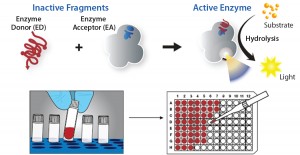
Figure 2: Examples from more than 30 bioassays developed as both potency and neutralizing antibody (NAb) assays for biosimilars; (a) response to three commercial insulin therapeutics (Humalog, Novolog, and Lantus) in a cell-based functional assay that measure activation of human insulin receptor; (b) a GLP1 and Exendin-4 bioassay was developed to accelerate development of biosimilars to metabolic drugs using a cyclic AMP readout in live cells. (c) A bioassay developed for anti-TNFα drugs enables users to benchmark drugs such as adalimumab, infliximab, etanercept, golimumab, and certolizumab against their respective biosimilar molecules. (d) A simple assay determines potency of the antiinflammatory antibody Ustekinumab.
DiscoveRx has developed more than 750 CBAs relying on the native biology of target receptors to quantitatively measure biologic drug potency using validated PathHunter enzyme-fragment complementation (EFC) technology (Figure 1). The flexibility of this platform enables interrogation of a diverse array of therapeutic targets (Figure 2). Those include biosimilar targets important in diabetes management (GLP1, exendin, and insulin) and inflammation (anti-TNFα and ustekinumab). These assays provide a readout that is proximal to the target — in contrast to the distal readouts of phenotypic and reporter-gene assays — providing a highly specific signal for each drug tested. The elegance of this technology is emphasized by its use of a simple, homogenous protocol for each available assay, allowing for rapid assay readout in 24–48 hours.
In addition, there is a significant drive within the industry to move away from continuous culturing of cells to increase the efficiency of potency-assay development. Continuous cell culture for assay development and lot-release testing can significantly increase assay cost, reduce efficiency, increase variability, and add up to two hours per day of culture and documentation time in a quality environment.
To overcome those challenges, the biologics industry is moving toward the use of ready-to-assay, cryopreserved cells. Users thaw cryopreserved cells directly onto an assay plate and perform an assay within 0–48 hours of doing so. This approach completely eliminates the need for end users to maintain cells in continuous culture, to harvest flasks, or to count cells before running an assay. Despite the fact that these cells are not cultured before an assay, they are optimized to ensure predictable performance immediately after thawing. This approach has been pioneered by both Genentech/Roche’s and Amgen’s potency assay development teams for developing bioassays in QC and lot-release testing (1, 2).
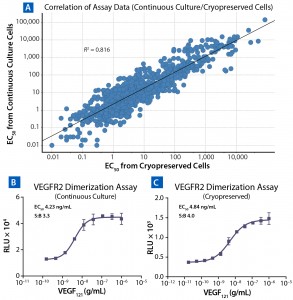
Figure 3: Comparing results for assays run with cells in continuous culture and cryopreserved ready-to-assay cells; (A) correlation of calculated EC50 from 842 cell-based assays using cells in continuous culture (y axis) and cryopreserved ready-to-assay format (x axis); a line of best fit includes an R2 value. To compare VEGFR2 assay conducted on cells in continuous culture (B) and cryopreserved ready-to-assay cells (C), cultured cells were lifted and plated with ligand for 16 h, then incubated with detection reagent; cryopreserved ready-to-assay cells were directly plated with ligand for 16 h before addition of detection reagent.
With deep experience in developing and optimizing cryopreserved ready-to-assay cells from continuous culture cell lines for more than 500 distinct assays, DiscoveRx has demonstrated broad-scale application of the approach. An oftenasked question is whether cryopreserved cells perform comparably to continuously cultured cells. Figure 3A compares data obtained using both assay formats, with EC50 derived from continuous-culture cells on the y axis and cryopreserved ready-to-assay cells on the x axis. For an overwhelming majority of targets, these two formats perform well and provide equivalent responses, as determined by an R2 of 0.814.
Figures 3B and 3C compare raw data generated through using continuously cultured cells and cryopreserved ready-to-assay cells in a VEGFR2 assay. Here, continuous culture cells were lifted, counted, and plated along with the ligand (VEGF121), then incubated for 16 hours (Figure 3B). Cells from the same single-cell–derived clone were frozen at an early passage and then used in a ready-to-assay format, in which they were thawed and plated directly with ligand onto an assay plate, followed by a 16-hour incubation (Figure 3C). Both assays produced comparable results in terms of signal-to-background ratio and EC50 for VEGF121.
With comparable functional output, the advantages of cryopreserved ready-to-assay cells become increasingly evident as noted by both Genentech and Amgen (1, 2). Use of such cells significantly increases
- efficiency (fewer personnel needed for cell maintenance and media preparation)
- data consistency and assay reproducibility (all cells in a given bank are frozen at the same passage number)
- operational flexibility (assays can be performed as and when needed) • cost savings (culture media and reagents)
- ease of assay development and method transfer to global sites (handling of cells during continuous culture can account for many transfer problems).
Ultimately, those features improve overall assay success and significantly reduce timelines for assay development and implementation. However, using a bank of frozen, ready-to-use cells requires proper qualification of those critical reagents to ensure long-term success. A recent paper by industry scientists and consultants offers guidelines for the proper creation, storage, and use of cryopreserved cell banks in quality environments (3). DiscoveRx has strictly adhered to those guidelines. Each lot of cryopreserved cells goes through robust QC checks, the results of which are provided in a detailed certificate of analysis (C of A) including full traceability to the original cell bank.
Case Study: Bevacizumab Bioassay
The power of PathHunter EFC technology for potency-assay development is illustrated by a bioassay developed for the angiogenesis inhibitor, bevacizumab. Its mechanism of action is to inhibit the growth of blood vessels in tumors by binding to vascular endothelial growth factor A (VEGF-A), preventing that protein from activating VEGFR2, the primary VEGF receptor found on endothelial cells. VEGF-A–induced activation of VEGFR2 promotes proliferation of endothelial cells, so proliferation of primary human umbilical vein endothelial cells (HUVECs) has been a traditional endpoint used in bevacizumab characterization (4, 5).
Figure 4: VEGF-A is known to cause homodimerization of VEGFR2 (KDR) as the first step in the activation cascade of these receptors. Anti-VEGF-A antibodies such as Bevacizumab prevent that dimer formation, leading to inhibition of VEGF-A–dependent signaling. (A) Testing of VEGF-A and bevacizumab in the HUVEC proliferation assay provides a functional readout of VEGF-A inhibition. (B) VEGFR2 homodimer bioassay treated with VEGF-A and bevacizumab; the latter displays a robust and precise response to both agents, with EC/IC50 results comparable to those obtained with the HUVEC proliferation assay. (C) The VEGFR2 assay is also highly tolerant of matrix (pooled normal human serum) concentrations as high as 90%.
Figure 4A shows an example of bevacizumab inhibiting VEGF-stimulated proliferation of HUVEC cells with an MTT dye-based readout. That assay is time-consuming (three to four days) and uses primary HUVECs, which can be highly variable in their performance for passage numbers, lots, and vendors because of donor heterogeneity. Additionally, culturing such cells is significantly challenging, with a risk of high variability and many failed runs. Furthermore, using cell proliferation as the assay endpoint reduces specificity because a number of growth factors can induce proliferation.
Alternatively, the PathHunter bevacizumab assay targets an early event in the VEGFR2 activation cascade. This assay quantifies VEGF-A–induced homodimerization of the VEGFR2 receptor, which is an early step in the receptor’s activation cascade. This activation event is blocked by the presence of anti-VEGF-A antibodies such as bevacizumab. As Figure 4B shows, VEGF-A stimulates VEGFR2 dimerization at an EC50 consistent with the reported ED50 for VEGF-induced proliferation in HUVECs of 1–6 ng/mL (4, 5). In addition, dose-dependent inhibition of dimerization by bevacizumab produces an IC50 of ~39 ng/mL, which is comparable to the published ED50 of 50 ng/mL for bevacizumab in a HUVEC proliferation assay (6).
The PathHunter method for testing bevacizumab potency has several advantages over HUVEC assays. It takes significantly less time (results obtained <24 hours rather than >96 hours), which increases assay throughput. The simple add-and-read protocol of the PathHunter assays uses ready-to-assay cryopreserved cells, bringing with them all the advantages discussed above. This assay is also extremely robust with high accuracy and precision and a larger assay window than the HUVEC proliferation assay (compare Figures 4A and Figure 4B). Additionally, good matrix tolerance (Figure 4C) enables the use of this assay in testing clinical samples for neutralizing antidrug antibodies.
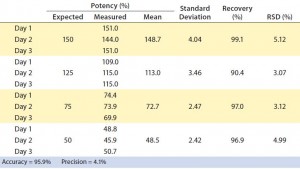
Table 1: Parallelism and relative potency of a reference standard can be measured with PathHunter assays (see Figure 5). The VEGFR2 dimerization assay was tested with four potency conditions from 50% to 150% and compared with a reference standard (100%).
To assess the suitability of the assay for potency testing, we performed an assay qualification exercise over three days. Relative to a reference sample of 100% potency, we tested samples of varying potency ranging from 150% to 50%, according to guidelines from the International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use (7, 8). As Table 1 shows, the assay was found to be very accurate (95.9%) and precise (4.1%) over the threeday exercise, with a relative standard deviation (RSD) <6%. To demonstrate linearity of sample measurements, we plotted measured against expected relative potencies (Figure 5A) and calculated the slope and R2 values, arriving at a respectable R2 value of 0.985.
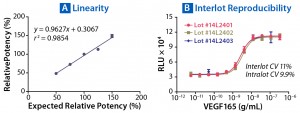
Figure 5: Parallelism and relative potency of a reference standard can be measured with PathHunter assays (see Table 1). (A) Measured relative potencies are plotted against expected relative potencies. The assay performed with high degrees of accuracy, precision, and linearity. (B) Variation in EC50 of VEGF165 was determined from three independently manufactured VEGFR2 bioassay lots. Excellent reproducibility is observed both within individual lots and among lots, with CVs <11%.
Finally, we created and tested three independent lots of ready-to-use PathHunter VEGFR2 dimerization cells to show the reproducibility of their response to the ligand VEGF-165. As Figure 5B shows, intralot and interlot variability met our acceptance criteria, showing <15% CV in EC50 between lots and 9.9–11% CV within lots (data not shown). Based on our results, PathHunter VEGFR2 dimerization cells performed well for potency and stability assays, as well as potentially for neutralizing-antibody assays, with a simple, robust, and reproducible ready-to-assay protocol.
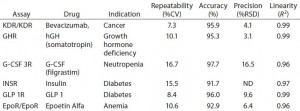
Table 2: Examples of PathHunter assays validated for potency and lot-release use; performance metrics are listed for six different therapeutic targets that are important in several therapeutic areas. All six assays met standard acceptance criteria for repeatability, accuracy, precision, and linearity.
Table 2 lists additional examples of PathHunter bioassays that are suitable for potency applications. These include bioassays for biosimilar drugs such as growth hormone, insulin, and granulocyte colony-stimulating factor (G-CSF), among others. All represent robust assays as demonstrated by their repeatability, precision, accuracy, and linearity values.
The Need for Speed
With increased global emphasis on development of innovator biologics and biosimilars alike, and with multiple developers targeting the same molecules, there is a clear race to be the first to submission for any molecule. This is driving the need for specific bioassays to accelerate and shorten their development timelines. Availability of commercial ready-to-assay cryopreserved cells in a qualified kit will enable developers to skip difficult and time-consuming method development and move on directly to assay validation for potency and neutralizing-antibody assays. For biosimilar developers, the target specificity and robust assay performance demonstrated by the examples shown herein are also important factors in establishing similarity with respect to quality and efficacy between their followon molecules and the innovator products they are meant to replace.
References
1 Gazzano-Santoro H, et al. Ready-to-Use Cryopreserved Primary Cells: A Novel Solution for QC Lot Release Potency Assays. BioProcess Int. 12(2) 2014: 26–38.
2 TerWee JA, et al. Increased Consistency and Efficiency in Routine Potency Testing By Bioassay with Direct Use of Cryopreserved (Ready-to-Plate) Cells. J. Immunol. Meth. 370(1–2) 2011: 65–74.
3 Menendez A, et al. Recommendations for Cell Banks Used in GXP Assays: Preparation, Characterization, and Storage. BioProcess Int. 10(1) 2012: 26–40.
4 Montesano R, Pepper M, Orci L. Angiogenesis In Vitro: Morphogenetic and Invasive Properties of Endothelial Cells. News Physiol. Sci. 5(2) 1990: 75–79.
5 Ferrara N, et al. Purification and Cloning of Vascular Endothelial Growth Factor Secreted By Pituitary Folliculostellate Cells. Meth. Enzymol. 198, 1991: 391–405.
6 Ebbers HC, et al. Measures of Biosimilarity in Monoclonal Antibodies in Oncology: The Case of Bevacizumab. Drug Disc. Tod. 18(17–18) 2013: 872–879.
7 ICH Q6B: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. US Fed. Reg. 18 August 1999: 44928; www.ich.org/fileadmin/Public_ Web_Site/ICH_Products/Guidelines/Quality/Q6B/Step4/Q6B_Guideline.pdf.
8 ICH Q2(R1): Validation of Analytical Procedures: Text and Methodology. US Fed. Reg. 1 March 1995: 11260; www.ich.org/fileadmin/Public_Web_Site/ICH_Products/ Guidelines/Quality/Q2_R1/Step4/Q2_R1__ Guideline.pdf.
Corresponding author Jane Lamerdin, PhD, is R&D director; Hanako Daino-Laizure, PhD, is a principal R&D scientist; Neil W. Charter, PhD, is director of LeadHunter discovery services; and Abhishek Saharia, PhD, is marketing director at DiscoveRx Corporation, 42501 Albrae Street, Fremont, CA 94538; 1-510-771-3555; jlamerdin@ discoverx.com; www.discoverx.com. Avastin is a registered trademark of Roche/ Genentech. PathHunter is a registered trademark of DiscoveRx Corporation. Humalog is a registered trademark of Eli Lilly and Company. Novolog is a registered trademark of Novo Nordisk. Lantus is a registered trademark of Sanofi-Aventis.
