Niche Disease: CAPS by Cheryl Scott Cryopyrin-associated periodic syndromes (CAPS) are rare, inherited, autoinflammatory diseases with similar genetics and overlapping symptoms. The group includes three subtypes: familial cold autoinflammatory syndrome (FCAS); Muckle-Wells syndrome (MWS); and neonatal-onset multisystem inflammatory disease (NOMID), also called chronic infantile neurologic cutaneous articular (CINCA) syndrome. The cryopyrin protein is critical to production of the inflammatory cytokine interleukin-1β (IL-1β), which triggers inflammatory response when it binds to inflammatory cells. Genetic alterations in cryopyrin cause IL-1β overproduction, resulting…
January 2016
The Case for a Standardized Assay to Test Suitability of Single-Use Systems in Cell Culture Applications
Increased commercial use of single-use systems (SUS) for large-scale biopharmaceutical production creates the need for consensus on industry best practices and standards for materials in SUS components. End users and suppliers are beginning to develop a shared vision of industry needs in such areas (1, 2). For example, highly visible efforts to harmonize extractables testing include contributions from groups such as the BioPhorum Operations Group (BPOG), Bio-Process Systems Alliance (BPSA), Parenteral Drug Association (PDA), ASTM, and ISPE. In addition to…
Hiring and Staffing in Biopharmaceutical Manufacturing: Five-Year Trends Indicate Difficulty in Filling Positions
The biopharmaceutical manufacturing industry continues to expand rapidly, with growth in revenue for both suppliers and drug innovators averaging about 15% per year. Understandably, much of the industry’s recent focus has been on the pace of technological improvements that have boosted productivity and performance. But another issue is becoming just as critical: The flow of skilled staff able to keep up with the industry’s growth isn’t keeping pace. Results from our 2015 annual industry study suggest that the hiring market…
Development, Qualification, and Application of a Bioreactor Scale-Down Process: Modeling Large-Scale Microcarrier Perfusion Cell Culture
Qualified scale-down models of large-scale cell culture processes are essential to conducting studies for applications such as investigating manufacturing deviations, enhancing process understanding, and improving process robustness. For example, scale-down models can be used for raw material investigations as well as evaluation and qualification of new good manufacturing practice (GMP) cell banks for manufacturing implementation. Process characterization studies are performed also with qualified scale-down models to improve process consistency (1, 2). Often it is impractical to conduct investigational studies at…
Heading for a CHO Revolution: The Need for Cell Line Engineering to Improve Manufacturing Cell Lines
The first recombinant protein licensed for use by the United States Food and Drug Administration (US FDA) was human insulin in 1982 (1). That approval was followed in 1987 by the development of tissue plasminogen activator (tPA), the first complex glycosylated protein generated in mammalian cells to be licensed for therapeutic use. Since then, this area of biology has rapidly expanded in clinics: The FDA approved an average of 15 new biological entities every year between 2006 and 2011 (2).…
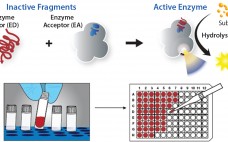
Accelerating Biologic and Biosimilar Drug Development: Ready-to-Use, Cell-Based Assays for Potency and Lot-Release Testing
With the drug industry’s expanding emphasis on biologics, the need for robust cell-based assays has grown at all stages of development. Requirements for efficacy, quality, and potency testing often demand a complex set of bioassays and/or cell-based assays for new therapeutics or biosimilars. Developers of the latter have found this need for cell-based assays to be particularly challenging. Commercially available, ready-to-use cell-based assays provide a robust functional response from specific therapeutic targets. They can significantly shorten assay development time while…
Ask the Expert: FOLDTEC Refolding of Biopharmaceuticals A Case Study of Recombinant Thrombin
with Dr. Andreas Anton and Dr. Sebastian Schuck Poorly soluble substances form aggregated inclusion bodies (IBs) in microbial cells containing incorrectly and/or incompletely folded target proteins. Wacker Biotech, a full-service contract manufacturer of biopharmaceuticals based on microbial systems, has introduced FOLDTEC refolding technology for bioengineered therapeutic proteins. The proprietary platform uses specifically developed and optimized bacterial strains and a patented, antibiotic-free expression system. In a BPI webinar on 9 November 2015, Wacker’s director of bioprocess development (Andreas Anton) and head…
Elucidation: Strict, but Flexible, Industrial Automation for Biopharmaceutical Manufacturing
Biopharmaceuticals are the fastest growing sector of the pharmaceutical industry, making up about 20% of the market, with annual growth rates of about 8% (double that of more traditional pharmaceutical sectors). To increase capacity and uphold stringent quality and regulatory demands, manufacturers often reassess their operational and technology strategies while focusing on rising manufacturing costs and the pressure of delivering cost-effective new drug products. Bioproduction can range from small batches to low-cost, high-volume campaigns. Few manufacturers have the required in-house…