As editors, we are fortunate to have the opportunity to listen to biopharmaceutical developers and innovators discuss the intricacies of their work. Typically, we find that the best discussions come from asking two fundamental questions: What need did you observe in the industry that drove your work, and what technologies would help you do your job better? Over the past five years or so, the answers have shifted. Cell and gene therapy (CGT) innovators are focusing on increasingly complex diseases…
April 2021 Featured Report
Untapped Potential of Tissue Engineering: The Three Obstacles Holding It Back
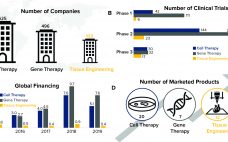
Regenerative medicine is the interdisciplinary field comprising tissue engineering, cell therapy, and gene therapy. These biopharmaceutical modalities, also referred to as advanced therapies, are growing rapidly, characterized by groundbreaking therapeutic advances that have the potential to change how healthcare providers deliver care. As Figure 1 shows, cell and gene therapies have gained traction over the past decade, as evidenced by large increases in investment and the number of marketed products. By contrast, tissue engineering investment and product commercialization has lagged…
The Difficulties of Manufacturing Cell and Gene Therapies At Scale
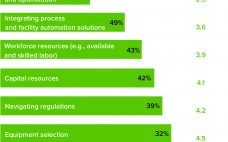
From large-scale manufacturing of one-size-fits-all blockbusters to small-scale processing of personalized therapies, the biopharmaceutical industry has undergone a revolution over the past decade. Among the standout milestones is the development of advanced therapy medicinal products (ATMPs). More than 1,000 of these research-intensive therapies are progressing through clinical trials toward potential commercial manufacturing. Cell and gene therapies (autologous and allogeneic) are targeted for many incurable diseases and conditions, including autoimmune disorders and cancers. Despite the excitement about ATMP potential, developers and…
Manufacture and Regulation of Cell, Gene, and Tissue Therapies: Part 2 — Regulatory Guidances
The US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the Medicines and Healthcare Products Regulatory Agency (MHRA), and Japan’s Pharmaceutical and Medical Devices Agency (PMDA) all offer support and guidance for developers of advanced therapy medicinal products (ATMPs). Some agencies have issued guidelines to help companies through different stages of product development — from research and development to marketing authorization and postauthorization activities. Such guidelines are updated regularly as more knowledge becomes available from the development and…