In highly regulated environments, such as the pharmaceutical industry, remaining GxP compliant while efficiently delivering your products to a competitive market is a challenging task, particularly when Western blot imaging is involved. While this can often be a tricky and time-consuming task, our Amersham ImageQuant™ 800 GxP imaging system and software streamlines the process. It provides simple solutions that support data traceability, accountability, and integrity. Confident decision making and GxP compliance go hand in hand with high-quality imaging. In this…
Sponsored Content
Optimization of an Anion Exchange Method for the Large-Scale Production of Plasmid DNA for Gene Therapy and DNA Vaccine Applications
This webcast features: Jenny England, Staff Scientist, Innovation & Applications, Thermo Fisher The advancement of gene therapy and plasmid DNA (pDNA) vaccines has highlighted the need for the development of a large-scale purification process to produce high purity plasmid DNA. Plasmid DNA serves as a foundation to produce viral vectors such as adenoassociated virus (AAV) or lentivirus (LV), a template for mRNA production during the in vitro transcription reaction and can be used directly as a pDNA vaccine. The typical…
Advancing Sterile-Connection Technology for Low-Volume Fluid Transfer
Biomanufacturers traditionally have relied on laminar-flow hoods or thermal tube welding to establish connections for small-format bioprocesses, including those for cell and gene therapy (CGT) production. But such strategies can introduce processing risks and can pose operational challenges. In October 2021, Troy Ostreng (senior product manager at CPC) described how his company’s MicroCNX sterile connectors could meet biomanufacturers’ needs for low-volume applications. Ostreng’s Presentation Welding tubes and forging open connections under laminar hoods raise several disadvantages for small-format bioprocesses. Processing…
Increasing productivity in cell and gene therapy bioprocessing with fast and reliable immunoassays
Cell and gene therapy manufacturers need to ensure the safe delivery of their products and rely on fast and reliable immunoassay platforms to do so. This white paper gives an overview of the viral vector landscape and the requirements for immunoassays in viral vector bioprocessing analytical workflow. It also shares case studies from viral vector labs that are using the Gyrolab technology to deliver fast and reliable results. Increasing productivity in cell and gene therapy bioprocessing with fast and reliable…
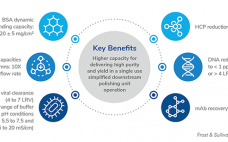
3M™ Polisher ST — The Next Frontier in Downstream Processing, a Frost & Sullivan White Paper
Advancements in next-generation therapies—Paving the way for novel bioprocessing solutions. Learn about 3M Polisher ST technology which utilizes a guanidinium-functionalized polyamide membrane protected by a Q functionalized non-woven material. This Frost & Sullivan whitepaper explains how the technology demonstrates the viability of replacing the depth filtration and anion exchange chromatography (AEX) steps to achieve a simplified and cost-effective process. The platform is designed to reduce process and product related impurities and offer robust performance across a wide range of process…
Analytical Ultracentrifugation for Profiling AAV Gene Therapies: Where Are We Now?
This webcast features: Yijun Huang, Scientific Fellow, WuXi Advanced Therapies Adenoassociated virus (AAV) is often the vector of choice for gene therapies due to their low immunogenicity, ability to promote long-term gene expression, and capsid tropism-dependent tissue specificity. Representing around 37% of the current advanced therapies market, there is an ongoing demand for robust quality lot release programs capable of supporting the large patient cohorts involved in clinical trials. Along with critical quality attributes such as titer, identity, and potency,…
Scale-Down Optimization to Scale Up Success
As immune cell therapy advances to address new indications, the need for rapid development of robust manufacturing processes becomes increasingly important. Early process optimization sets the stage for clinical and commercial manufacturing and plays a foundational role in decreasing time to market and lowering COGS. This application note describes the streamlining of T-cell expansion optimization using a DOE-based approach in a semi-automated, controlled multi-parallel setup of the Sartorius T-Cell Exploration and Characterization Solution. Download to learn about: Rapidly screening media…
Hot off the Press: Quality by Design for AAV manufacture
Chemistry Manufacturing and Controls (CMC) for gene therapies is one of the biggest obstacles when moving towards regulatory approval and presents a significant risk to the success of new gene therapy drug candidates today. A key aspect to the CMC documentation of such complex biological products is the application of the Quality by Design (QbD) principle: A rationale of quality being achieved by process design rather than relying on final quality testing alone. The work presented here provides a framework…
Container Closure Integrity: Testing of Bottles Used for Cell Therapy Applications
This webcast features: Eric Isberg, Vice President, Life Sciences, Savillex Container integrity is critical when storing and shipping cell therapy products to ensure sterility. The security and durability of the container sealing region(s) should be weighed against usability of the container in clinical settings common to cell therapies. A study was performed to evaluate and quantitatively measure the associated leak rate for the container closure integrity of fluoropolymer bottles per USP <1207> Package Integrity Evaluation – Sterile Products using helium…
MAb Quantitation: Protein A HPLC vs. Protein A Bio-Layer Interferometry
Rapid, accurate and cost-effective quantitation of monoclonal antibodies (MAbs) is essential for bioprocessing. High Pressure Liquid Chromatography (HPLC) and the Octet® are some of the commonly used techniques for MAbs titer determination. To ensure MAbs purification column efficiency, the dynamic binding capacity (DBC) of Protein A for Mab can be determined by loading feedstock onto the column until binding sites for the MAb become saturated and MAb begins to break through. An assessment of the relative merits of Protein A…