For all stages throughout the development, manufacturing and release of your biological product, Eurofins BioPharma Product Testing offers comprehensive, fully CGMP-compliant Viral Clearance Services. We have two dedicated clearance suites for extensive capacity and timely execution and reporting of your study results. And all of our assays are fully validated to meet ICH Q2 requirements. Why Choose Eurofins BioPharma Product Testing? We bring together leading experts in the industry with extensive scientific and regulatory experience. By supporting you from study…
Sponsored Content
The Viscosity Reduction Platform: Viscosity-Reducing Excipients for Protein Formulation
Protein viscosity is one of the major obstacles in preparing highly concentrated protein formulations suitable for subcutaneous (subQ) injection. Highly viscous protein solutions would require a significant force to be applied to the syringe for injection. As a result, the patient could experience a considerable amount of pain. In many cases, injectability would not be possible. When characterizing protein viscosity behavior, one can differentiate two different concentration regimes. At very low concentrations below about 75 mg/mL, proteins are rarely viscous.…
Optimized Expression Systems for Increased Productivity in CHO DG44 Based Cell Line Development
This webcast features: Dr. Rathangadhara Nammalwar, Team Lead, Cell Line Development for Protein-Based Therapeutics, Sartorius The Global Cell Line Development (CLD) services market is growing at an unprecedented pace. In 2020, the global revenues were about $700 million and are estimated to reach nearly $1.7 billion by 2028. Chinese hamster ovary (CHO) cells have been the most preferred host cell line for those CLD services, and they alone generated more than 50% of the global revenue in 2020. Key success…
Efficient and Scalable GMP Manufacture of AAV and LV with TransIT-VirusGEN® Reagent and Enhancers
This webcast features: Leisha Kopp, Applications Scientist, Mirus Bio Recombinant adenoassociated virus (AAV) and lentivirus (LV) are essential components of many gene and cell therapies designed to treat and potentially cure a vast array of acquired and heritable diseases. Thus, the need for large-scale manufacture of safe and effective viral vectors for the development of biotherapeutics has never been greater. In this webinar, we will examine critical optimization strategies that lead to simplified, defined workflows and enable higher AAV and…
Accelerating Discoveries for Viral Biology and Host Immunity with Advanced Cell Analysis Solutions
This whitepaper summaries recently published studies that incorporated the Incucyte® Live-Cell analysis platform and iQue® 3 Advanced High-Throughput Flow Cytometry. These state-of-the-art technologies were used to simplify workflows and provide rapid analysis solutions and enhanced throughput, aiding the discovery of new insights on the host-pathogen life cycle throughout the course of infection. Post-infection, these platforms were leveraged to monitor exposure, assess immune protection, and accelerate the development of anti-viral small molecules, neutralizing antibodies, and vaccines. The Incucyte® platform offers automated…
Spurring on Innovation in Gene Therapy Development
Gene therapies based on adenoassociated virus (AAV) vectors hold promise for treating myriad conditions. Immunogenicity remains a challenge for such products, however. With support from PerkinElmer, Roland W. Herzog (professor of pediatrics and Riley Children’s Foundation professor of immunology at the Indiana University School of Medicine) joined Nagendra Venkata Chemuturi (scientific director of global research for drug metabolism and pharmacokinetics, DMPK, at Takeda Pharmaceuticals) to deliver a BPI “Ask the Expert” presentation exploring strategies for minimizing immune responses to AAV-based…
Cell Media Analysis: A Critical Piece of the Bioprocessing Puzzle
This webcast features: Graziella Piras, Bioprocess Segment Director, 908 Devices Cell culture medium remains a key component of traditional monoclonal antibody (mAb)-based biologics as well as newer modalities such as gene and cell therapies manufacturing. As such, cell culture media development and optimization continue to be an important focus for the biopharmaceutical industry. Increased analytical capabilities have provided new insights into the relationship between cell culture media, the cells they support, and ultimately the outcome of the final product. That greater insight offers more…
A Strategic Approach to Selecting the Optimal Process Intensification Scenario
Current demands placed on the biopharmaceutical industry are pushing manufacturers toward process intensification, an approach that modifies unit operations or an entire manufacturing process to optimize efficiency. Three common intensification scenarios in upstream processing are seed-train intensification (usually at the n – 1 stage), concentrated fed-batch production, and dynamic perfusion (at the production bioreactor stage). In downstream processes, intensification strategies typically involve moving from single- to multicolumn chromatography. Biomanufacturers can realize several kinds of improvements from intensified processing, including reductions…
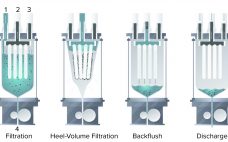
Comparing Single-Use Multicycle Cake Filtration with Depth Filtration: Eliminating the Downstream Bottleneck
Over the past few decades, single-use (SU) technology has increased bioproduction efficiency significantly, especially with the introduction of disposable bioreactors in upstream processing. To keep pace with major developments and increases in upstream capacity, downstream processes also must increase capacity and efficiency. However, cell harvesting and downstream processing continue to present bottlenecks in manufacturing (1). Typical clarification processes are composed of primary and secondary clarification steps, such as centrifugation followed by depth filtration, respectively (1). Two sets of SU depth…
Transfection Best Practices for AAV Gene Therapy Programs
As viral vectors continue to push gene therapy innovations closer to market, many researchers are setting their sights on optimizing transfection, the process of delivering corrective genetic material into cells. It’s not just a question of how to transfect them, but also how to do so efficiently and at high volumes. Approaches that work for one cell line might not perform well for others, and transfection protocols can have different implications for scalability and cost during production for clinical trials.…