Frozen storage of bulk drug substance (BDS) at temperatures at or below –70°C is common in the bioprocess industry. Storage at temperatures below –80°C can lead to spontaneous failure of typical BDS containers, particularly if container temperature is reduced rapidly (known as flash freezing). Most containers used to store BDS have glass-transition temperatures well above –196°C, and many containers structurally fail during rapid descent through glass transition. Even worse, such failures often are detected after a container is thawed, which…
Sponsored Content
Noncontact SONOFLOW®C0.55 Clamp-On Flow Meters and SONOCHECK®ABD06 Bubble Detectors
Noninvasive SONOFLOW clamp-on flow sensors and noninvasive SONOCHECK bubble detectors are designed for accurate and contamination-free upstream and downstream monitoring to fulfill regulatory goals within the process analytical technology (PAT) framework. Field-Proven Technology: SONOFLOW clamp-on flow sensors are designed for upstream and downstream bioprocess monitoring. These innovative sensors have integrated electronics that enable them to function without an external board or transmitter, providing a complete flow meter in a device the size of a small transducer. The systems can be…
Reduce Downstream Processing Costs for Monoclonal Antibodies: Switch to Tosoh’s Two-Step Platform
In this study, we showcase the benefits of Tosoh’s two-step process for the purification of monoclonal antibodies (MAbs) in comparison with the standard industrial process. Combining high-performance protein A capturing and a single polishing step on a salt-tolerant anion-exchange resin, we could reduce the downstream costs by 45% and increase production output by 58%. TOYOPEARL AF-rProtein A HC-650F is a high-capacity protein A resin for the purification of MAbs. This resin exhibits dynamic binding capacities (DBC) of 70 g/L at…
Who We Are
AbbVie is a global, research-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions. The company’s mission is to use its expertise, dedicated people, and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology, and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. Combining the focus of a biotechnology company with…
Giving You the Power for Clinical and Commercial Success
As a result of increased focus on customized medicine and continued emphasis on drug development for rare diseases, the biopharmaceutical industry continues to advance its methods for disease treatment by developing target-specific novel therapeutics. Formulations for these types of programs are complex and therefore present challenges in the design, scale-up, and manufacture of drug substances and drug products. Such challenges can delay getting critical products to patients. Contract development and manufacturing organizations (CDMOs) can help advance therapeutics and navigate production…
27 Years of Biologics Development and Manufacturing Experience
Avid Bioservices is a full-service, dedicated contract development and manufacturing organization (CDMO) focused on development and manufacturing of biopharmaceutical drug substances derived from mammalian cell culture. Avid’s biologics development and manufacturing services include process development and current good manufacturing practice (CGMP) clinical and commercial drug substance manufacturing, bulk packaging, release and stability testing, and regulatory submissions support. For early stage programs, the company provides various process development activities, including upstream and downstream development and optimization, analytical methods development, testing, and…
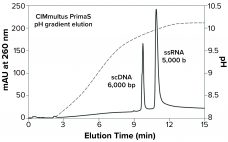
CIMmultus PrimaS: The Next-Generation Anion Exchanger for Today’s mRNA-Based Vaccines
CIMmultus PrimaS multimode ligand bioprocessing technology represents a new class of anion exchangers for fast, efficient, one-step purification of single-stranded mRNA at ambient temperature. DNA, dsRNA, and proteins are eliminated with a high-salt wash. Then ssRNA is fractionated by size in a pH gradient that removes short transcripts and fragments. Convective mass transfer and laminar flow through the technology’s monolithic architecture maintain high capacity, high resolution, and low shear stress — even at flow rates >5 column volumes per minute.…
Bridging the Gap from Process Development to CGMP Manufacturing for Gene Therapies
Gene therapy holds the promise of treating the unmet needs of patients who suffer from rare genetic diseases. An estimated 4,000 medical conditions are a result of gene disorders with no established targeted treatments. Gene therapy offers new options away from the conventional symptomatic approach to disease treatment as well as provides hope for real cures. At Catalent Cell & Gene Therapy, we understand the unique and complex nature of bringing a gene therapy to market, and we are dedicated…
Emergent Biosolutions CDMO Services
Emergent BioSolutions is a global life sciences company whose mission is to protect and enhance life. Through our specialty products and contract development and manufacturing services, we are dedicated to providing solutions that address public health threats. Through social responsibility, we aim to build healthier and safer communities. We aspire to deliver peace of mind to our patients and customers so that they can focus on what’s most important in their lives. By working together, we envision protecting or enhancing…
Optimal Time to Market for Life-Saving Biopharmaceuticals
Rentschler Biopharma is an international contract development and manufacturing organization (CDMO) dedicated to biopharmaceutical development and biomanufacturing for over 40 years. We are leading experts with an exceptional track record of over 100 different therapeutic protein formats. As a world-class solution provider, we ensure optimum time to proof-of-concept for clinical stages I and 2, as well as time to market for products in clinical stage 3. By accelerating timelines under consideration of economically attractive process options, we generate a competitive…